Abstract
Chronic kidney disease (CKD) continues to be a global public health problem. Globally, the prevalence of CKD is approximately 8–16% in the general population. Most patients with CKD advance to kidney failure and require dialysis or kidney transplantation. Screening for CKD, diagnosing CKD, treating CKD and its consequences to stop its progression, and renal replacement therapy (RRT) are all parts of comprehensive CKD care. A 28-year-old male presented with complaints of awareness of his heart beating, abdomen and lower limb swelling, and generalised body weakness for 2 days. His blood pressure was 222/147 mmHg on admission day. Six days post-admission, he displayed violent chest pain and dyspnoea, along with profound generalised body swelling. Laboratory studies revealed creatinine of 22.49 mg/dL (0.6–1.1), urea of 236.5 mg/dL (10.0–50), albumin of 2.15 mg/dL (3.8–5.1), potassium of 7.19 mmol/L (3.5–5.5), and haemoglobin of 6.2 g/dL (8.0–17.0). The diagnoses of uremic pericarditis, pulmonary oedema, hyperkalaemia, hypertensive emergency, and normochromic anaemia secondary to end-stage renal diseases were made. He qualified for the RRT. CKD is a serious, non-communicable disease that is commonly encountered in clinical practice in both developed and developing countries and needs the utmost attention. RRT is crucial for comprehensive CKD management; however, in resource-limited healthcare settings, RRT is non-accessible and non-affordable. The lack of RRT marks the mistreatment of patients with renal diseases by the global healthcare system. The author calls for designing new strategies that aim to ensure equitable accessibility and affordability for RRT globally.
Key Points
1. This manuscript aimed to shed attention on chronic kidney disease (CKD) as one of the most devastating non-communicable illnesses, which is regrettably overlooked in healthcare settings with inadequate resources.
2. Approximately 8–16% of the general population worldwide has CKD. Comprehensive CKD care includes screening for CKD, diagnosing CKD, treating CKD and its implications, halting its progression, and providing renal replacement therapy.
3. The worldwide healthcare system mistreats patients with renal disorders because it denies them access to renal replacement therapy when necessary. To guarantee accessibility and affordability for all patients who require it, new, robust, and transformational strategies are required on a global scale. Further, comprehensive CKD care strategies are required to fully combat all impacts of chronic renal disease.
INTRODUCTION
Kidneys are a vital pair of bean-shaped organs situated in the retroperitoneal space and positioned on either side of the vertebral column at the level of the inferior thoracic and superior lumbar vertebrae. The estimated weight of each adult human kidney is 120–170 g. The dimensions of each human’s kidneys are 12×6×3 cm. The normal functioning of kidneys leads to accurate body fluid composition, such as red blood cell mass; proper excretion of waste products from the metabolism of endogenous substances such as urea and xenobiotic substances; accurate regulation of electrolyte metabolism, such as calcium and phosphate balance; and the synthesising and secretion of hormones, such as erythropoietin. To perform these functions, human kidneys must receive an adequate blood supply, have intact innervation, and have normal and adequate nephrons. Chronic kidney disease (CKD) is one of the most common medical conditions that disturbs the integrity and functional capacities of humans’ kidneys. CKD refers to “structural or functional abnormalities of the kidneys for >3 months, as indicated either by kidney damage with or without decreased glomerular filtration rate (GFR), as defined by pathologic abnormalities markers of kidney damage, such as abnormalities in the composition of the blood or urine or abnormalities in imaging tests.”1,2 Moreover, CKD is also evident when ‘GFR<60 mL/min/1.73 m2, with or without kidney damage or albuminuria ≥30 mg per 24 hours’ for >3 months.2-4 According to worldwide kidney data, in 2019, 850 million people had kidney illnesses, of which 843.6 million had CKD.5 Globally, non-communicable diseases continue to increase, and they are among the prominent factors leading to CKD and its increased mortality and morbidity.3,4 For instance, the incidence of end-stage renal disease (ESRD) has been reported to be about 44.8% amongst patients with diabetes; 27.4% among patients with hypertensive and large vessel disease; 7.7% for patients with glomerulonephritis; 3.4% for patients with interstitial nephritis and pyelonephritis; 3.1% for patients with cystic, hereditary, and congenital diseases; 2.4% for patients with tumours and neoplasia; 2.2% for patients with secondary glomerulonephritis and vasculitis; approximately 4.9% for patients with miscellaneous conditions (such as those with HIV/AIDS); and for about 7.5%, the causes are unknown.6
The advancement of medical technologies has improved the diagnosis, prevention, and treatment of CKD. In the 1940s, dialysis technology was available.7 In 1954, the kidney was the first human organ to be transplanted.8 However, all these lifesaving, innovative medical technologies came with many ethical dilemmas related to the distribution of scarce resources.9 For many decades, the non-accessibility and non-affordability of renal replacement therapy (RRT) have been serious medical challenges.3,10,11 In fact, in resource-limited healthcare settings, the situation is still worse because the majority of patients with kidney diseases who need RRT are not able to access such therapy promptly, and many are not able to afford its payment.12 Here, a 28-year-old male who was diagnosed with ESRD complicated with uremic pericarditis, pulmonary oedema, hyperkalaemia, hypertensive emergency, and normochromic anaemia is reported. He qualified for RRT; however, due to inadequate resources and poor socioeconomic status, RRT was not provided to him.
CASE PRESENTATION
A 28-year-old male presented with complaints of awareness of his heart beating, swelling of the abdomen, swelling of the lower limbs, and generalised body weakness for 2 days. Four months prior to presentation, he was diagnosed with acute glomerulonephritis and severe hypertension. Moreover, 1 month prior, he developed painful abdominal swelling, awareness of his heart beating, dizziness, generalised body weakness, and swelling of the lower limbs, for which he visited a nearby clinic, which treated him and possibly improved him.
A pertinent physical examination revealed a fairly well-looking male who was afebrile. However, he had a moon-face appearance, conjunctiva pallor, a distended abdomen, a blood pressure (BP) of 222/147 mmHg, a pulse rate of 101 beats per minute, and a respiratory rate of 19 cycles per minute. Treatment interventions included medications such as intravenous (IV) lasix 40 mg once a day (OD), tablets of nifedipine 40 mg twice per day (BID), tablets of captopril 25 mg OD, and tablets of spironolactone 50 mg OD. Two days following admission, his BP remained high. However, the treatment plan was adjusted by replacing tablets of captopril 25 mg OD with tablets of lisinopril 20 mg OD for 1 month. Tablets of bisoprolol 5 mg OD for 1 month were added. Tablets of nifedipine, spironolactone, IV lasix, and metolazone, as well as oxygen therapy (2 L of O2), were continued.
Despite this treatment plan and the institution of those medications, the patient’s condition did not improve, as evidenced by a constantly raised BP in the range of 160/100 mmHg–230/140 mmHg, and visible difficulty breathing despite being on oxygen therapy. On 26 July, 2023, the patient displayed violent chest pain, dyspnoea, profound generalised body swelling, and body weakness. The chest pain was sharp in nature, and exacerbated by lying down in the supine position and turning his body while in the supine position. Leaning forward could relieve his pain. Physical examination revealed obvious facial swelling, abdominal swelling, bilateral non-pitting oedema, BP of 189/110 mmHg, pulse rate of 105/min, and oxygen saturation of 92–94% on an oxygen mask with no reservoir at a flow rate of 5 L/min. Despite being on oxygen, he had severe respiratory distress and a tachypnoeic status of 43 cycle per minutes. Auscultation of the lungs revealed diffuse crackles on the left and right lung. He had a heart murmur of 7th intensity that was heard without a stethoscope.
His emergent laboratory studies included a complete blood count and differential, liver function test, renal function test, serum electrolyte, and urinalysis (Table 1). Low haemoglobin levels (6.2 g/dL) prompted the transfusion of two units of packed red blood cells. With the diagnoses of uremic pericarditis, pulmonary oedema, hyperkalaemia, hypertensive emergency, and normochromic anaemia secondary to ESRD, the standard treatment plans were to offer him renal RRT. However, due to resource limitations, the patient continued to struggle. His conditions were only managed symptomatically with IV furosemide 120 mg BID, tablets of metolazone 10 mg OD, IV calcium gluconate 10%/10 mL/over 10 min bolus, and IV Actrapid 10 units in 30 mL of 50% dextrose. Nebulisation with salbutamol 5 mL every 6 hours, kayexalate 30 g orally four times a day, morphine 5 mg orally (5 mL every 8 hours), bisacodyl 10 mg tablets at night for 5 days, capsules of cudo forte (one capsule OD for 1 month), omeprazole, and continuation of oxygen therapy were prescribed. Frequent monitoring of his vital signs revealed a persistently high BP of >160 mmHg. Moreover, frequent serum electrolytes continued to reveal a high potassium concentration >5.5 mmol/dL. On 1 August, 2023, the patient reported marked improvement, and on 3 August, 2023, he voluntarily requested discharge. However, his BP remained high (180/110 mmHg), and his potassium levels also remained above 7 mmol/dL. However, these findings did not correlate with a visible improvement picture. The patient was discharged on oral antihypertensive medications with an appointment for a 2-week follow-up.
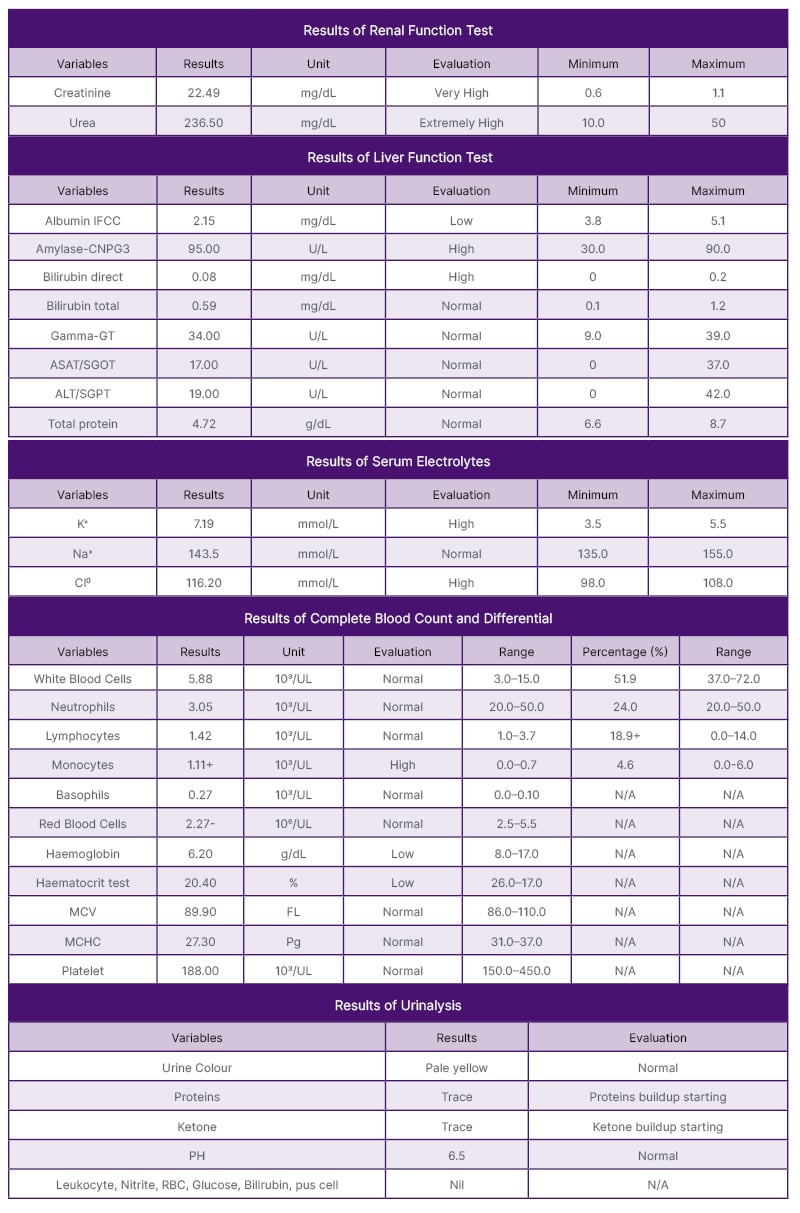
Table 1: Renal function test, liver function test, complete blood count and differential, serum electrolyte, and urinalysis.
ASAT/SGOT: aspartate aminotransferase/serum glutamic-oxaloacetic transaminase; ALT/SGPT: alanine transaminase/ serum glutamic pyruvic transaminase; Cl–: chlorine ion; FL: femtolitre; Gamma-GT: gamma-glutamyl transferase; IFCC: International Federation of Clinical Chemistry; K+: potassium ions; N/A: not applicable; Na+: sodium ion; MCHC: Mean corpuscular haemoglobin concentration; MCV: mean corpuscular volume; Pg: picograms.
DISCUSSION
This case report highlights CKD as one of the most serious non-communicable diseases. However, it is still a neglected condition in resource-limited healthcare settings.13 Populations at risk for developing CKD include people with previous comorbidities such as diabetes, hypertension, or any renal disease.14 Suboptimal control of these conditions and ineffective and non-efficient policies for tackling non-communicable diseases, including CKD, especially in developing countries, increase the mortality and morbidity associated with CKD.15
For clinical and epidemiological purposes, three variables are crucial criteria to ascertain the burden and choose appropriate management approaches for CKD. These include the presence of CKD for ≥3 months, the irreversibility of kidney damage, and the reduction of GFR. CKD denotes the progressive and irreversible loss of kidney function.1 Both acute and chronic conditions can provoke nephron loss, which may result in chronic renal insufficiency, chronic kidney diseases, and eventually ESRD and death, as shown in Figure 1. Elevated serum creatinine, proteinuria, and a decline in the GFR may signify the continuum of CKD.16 Continuous loss of the kidneys’ nephrons is the hallmark mechanism leading to the development of CKD. Human kidneys have around 1 million nephrons. The GFR in each kidney is about 120 mL/min. Thus, the single nephron glomerular filtration rate is about 60 nanolitres per minute. Any conditions leading to the loss of nephrons provoke compensatory mechanisms to take place in undamaged and less damaged nephrons, which results in hypertrophy of nephrons. In intact nephrons, single nephron glomerular filtration rate tends to increase; thus, theoretically, GFR is maintained at normal. Such adaptation is possible because of humeral and hormonal changes. The continuous damage to kidney nephrons leads to a reduction in GFR. According to Kidney Disease: Improving Global Outcomes (KDIGO) working group, a GFR of >90 mL/min (1.73 m2) may indicate normal kidney functions; a GFR of 60–89 may indicate mildly decreased kidney functions or chronic renal insufficiency; a GFR of 45–59 may mark mildly to moderately decreased kidney functions; a GFR of 30–44 may indicate moderately to severely decreased kidney functions; a GFR of 15–29 may designate severely decreased kidney functions; and a GFR of <15 may confirm a kidney failure.3 The whole loss of the kidney’s function indicates ESRD.
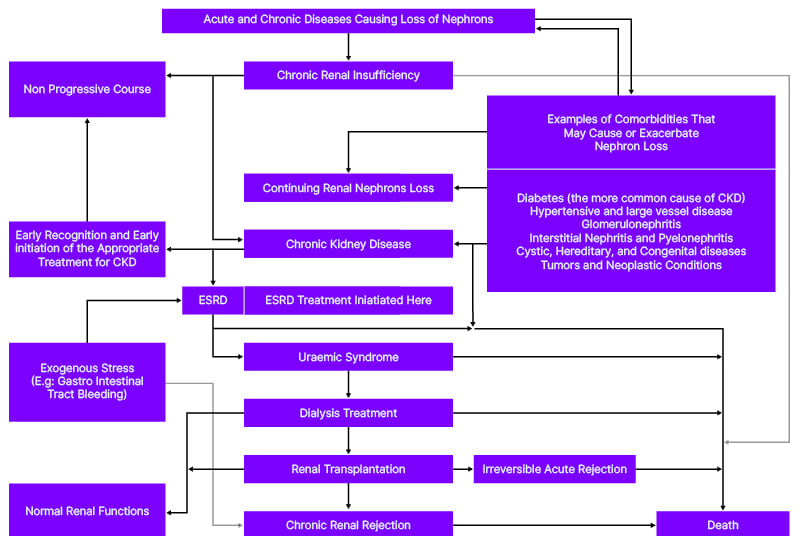
Figure 1: Stepwise progression of chronic kidney disease and possible outcomes.
CKD: chronic kidney disease; ESRD: end-stage renal disease.
Clinical manifestations of CKD vary and depend on the extent of kidney damage. Accumulation of waste products, deficiencies of some crucial body chemicals, and the development of complications lead to the apparent appearance of various signs and symptoms that would aid in the diagnosis of CKD and, in fact, the status of its progression.6 Uremic syndrome affects multiple human body systems.17 The presence of uremic symptoms may indicate ESRD. The physician treating the patient with CKD must be able to recognise all conditions that require emergent attention to avoid adverse outcomes. Hyperkalaemia, pulmonary oedema, and acute pericarditis are dangerous complications of CKD that were diagnosed in this patient. He needed emergent or urgent dialysis to prevent cardiac arrest and other catastrophic outcomes, but because of resource limitations, dialysis was not offered to him. The differential diagnosis of pericarditis includes pneumonitis and pleuritis. Respiratory distress and pulmonary oedema are common for CKD patients; these are commonly seen in patients with CKD who develop ESRD.17 This patient had respiratory distress, which signified acid-base metabolic disturbances. Arterial blood gas tests would help to ascertain the presence of such disturbances; however, the laboratory setting in which this patient was treated was not equipped for doing such tests.
Serum concentrations of creatinine and blood urea nitrogen (BUN) can be used to predict the development of uremic syndrome and to diagnose impaired renal function. The normal range of BUN is 5–20 mg/dL, while the normal range of serum creatinine is 0.9–1.3 mg/dL (adult males) and 0.6–1.1 mg/dL (adult females).18-20 Serum creatinine is a more specific and sensitive indicator of kidney disease than BUN. However, in chronic renal disease, both BUN and creatinine are relevant to evaluating renal problems because the BUN-to-creatinine ratio provides greater information. The normal range of the BUN-to-creatinine ratio is 10:1–20:1. It is unusual to develop uremic syndrome if the BUN is <60 mg/dL and the serum creatinine level is 8 mg/dL. However, the likelihood of the occurrence of uremic syndrome is high when the BUN level is >100 mg/dL or the serum creatinine level is >12 mg/dL. This patient had a creatinine concentration of 22.49 mg/dL (0.9–1.3) and a BUN concentration of 236.5 mg/dL, which correlated with his uremic syndrome manifestations and complications. Having had pericarditis means that the accumulation of toxic substances (urea) responsible for uremic syndrome is chronic. The pain associated with pericarditis is exacerbated by lying flat and breathing, but such pain is relieved by sitting upright and leaning forward. Fibrosis that occurs as a result of inflammatory healing is responsible for such manifestations. The formation of fibrosis takes a long time. Thus, the occurrence of uremic pericarditis may firmly connote the chronicity of CKD in this patient.
Albumin consists of about 55–60% of the measured serum proteins.21 Albumin is the main performer in maintaining normal oncotic pressure within the human body. Oncotic pressure is one of the determinants that maintain Starling’s forces. Oncotic pressure plays a role in sucking fluid from interstitial space back to the bloodstream. Albumin loss in patients with CKD leads to hypoalbuminemia, and eventually, an overwhelming accumulation of fluid within the interstitial body compartment. This patient had low serum albumin concentrations (2.15 mg/dL), which explained his generalised body swelling and the presence of pulmonary oedema. Likely, inflammation was the cause of hypoalbuminemia in this patient. Having had a past medical history of acute glomerulonephritis connotes this claim. He would have benefited from a vasculitis workup and urine sediment exam; however, these investigations were not done. Inflammatory status increases vascular permeability and the escape of serum albumin from the vascular compartment, causing expansion of the interstitial space and the increase of distribution volume of albumin.22 He had non-pitting oedema, which would confirm the possibility of albumin accumulation in his interstitial fluid compartment secondary to inflammation because proteins have a higher density than water. Atherosclerotic lesions, vascular calcification, vascular senescence, myocardial fibrosis, and calcification of the heart valves are all outcomes of the systemic, chronic proinflammatory condition that CKD generates.4 Cardiovascular events pose a significant danger to patients with CKD.23-25 In patients with CKD Stage 4–5, cardiovascular events account for almost half of the cases. Studies have shown that patients with ESRD (Stage 5) and advanced CKD (Stage 4) have cardiovascular mortality rates of approximately 40–50%, while controls with normal kidney function have cardiovascular mortality rates of 26%.4,25 This patient had severe respiratory distress, tachypnoeic status, bilateral diffuse lung crackles, and a heart murmur of 7th intensity, confirming cardiovascular events. All drugs used to treat inflammatory conditions can be dangerous bombs that destroy human kidneys. Apart from the recommendation that these drugs should be stopped for patients with CKD, all available guidelines for managing patients with CKD have not addressed how to treat inflammatory conditions in these patients. Further studies are required to investigate what works and what does not work to alter the chronic proinflammatory conditions that CKD generates.
This patient had serum potassium of 7.19 mmol/L (3.5–5.5), which indicated severe hyperkalaemia. Hyperkalaemia in CKD may indicate the chronicity of CKD and the extreme loss of nephrons. Potassium excretion depends on renal tubular secretion. Aldosterone and distal tubular fluid flow rate are major determinants of potassium excretion. Healthy kidneys’ nephrons excrete about 90–95% of ingested potassium, and the rest (5%) is excreted via the stool. An increase in aldosterone levels and increased fluid flow in the distal tubules are important adaptation mechanisms that support patients with CKD in continuing to excrete potassium from the human body. However, profound loss of human kidney nephrons and reduction of GFR to less than 10 mL/L leads to the accumulation of potassium. Hyperkalaemia may be due to the redistribution of potassium between the intracellular and extracellular compartments. A decline in GFR to <5 mL/min provokes the excretion of about 20–50% of potassium ions in the stool. This fact may justify the use of bisacodyl as an enhancer of potassium excretion in conjunction with enhanced stool excretions. However, currently, there are no well-published randomised studies that have investigated the effectiveness and safety of bisacodyl in regards to treating hyperkalaemia in patients with ESRD. Moreover, measuring stool potassium concentration may be a good and easy diagnostic test for detecting CKD progression; however, studies regarding this claim have not been done.
Commonly, patients with CKD develop normochromic anaemia due to the inadequate formation of erythropoietin. Anaemia increases the morbidity and mortality associated with CKD.26,27 Erythropoietin is a hormone formed in the kidneys. Its function is to ensure red blood cell maturation in the bone marrow.26 This patient had a haemoglobin level of 6.2 g/dL (8.0–17.0) and a mean corpuscular volume of 89.9 FL (86.0–110.0). Having had generalised body weakness would also correlate with the findings of normochromic anaemia. Researchers have discovered erythropoietin-stimulating agents and have made them the standard treatment for anaemia in patients with CKD.28 Epoetin alfa 50 200 U/kg/week by subcutaneous IV 2–3/week or darbepoetin alfa 20–40 mg delivered subcutaneously weekly have been approved for the symptoms control associated with anaemia of CKD.29 This patient required these medications; however, they were unavailable. Thus, the transfusion of packed blood cells was an option for controlling his anaemia. This practice somehow contrasts the KDIGO recommendation statements on red cell transfusion to treat anaemia in CKD, which state that, if possible, red cell transfusions should be avoided to minimise the general risks related to their use.30
Comprehensive management for CKD encompasses screening for CKD, diagnosis of CKD, treating CKD and its complications to prevent CKD development and progression, and RRT.3,6,29,31 All patients with comorbidities known to eventually complicate CKD should be routinely screened. Early recognition of CKD via screening can promote the early initiation of appropriate treatment.32 There is a higher likelihood of a non-progressive CKD course in such a case. Thus, this patient would have benefited from CKD screening services because he had a past medical history of acute glomerulonephritis and severe hypertension. In fact, affirming CKD diagnosis without prior 3-month laboratory findings of this patient is anecdotal. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are crucial for preventing CKD progression because of their effects on reducing long-term remodelling of the nephrons of patients with CKD.29 However, they are contraindicated in ESRD due to the risk of causing hyperkalaemia. From 21–25 July, 2023, among the prescribed medications for this patient were aptopril 25 mg OD and Lisinopril 20 mg OD. Due to a lack of meticulous CKD screening services, on admission day, the diagnosis of ESRD was not made for this patient. It is not clear whether the prescribed oral angiotensin-converting enzyme inhibitors were the cause of the violent deterioration (specifically, hyperkalaemic state) in this patient. The appropriate time for which the diagnosis of CKD would have been made for this patient is also not clear. His high BP of 222/147 mmHg and some features of vital end organ damage indicated a hypertensive emergency that needed meticulous management with appropriate IV antihypertensive medications. IV Lasix was given to this patient, but it was not enough; other intravenous antihypertensive medications would have been given to him if they were available. About 5–10% of adult patients with elevated BP have secondary hypertension.33 Attention should be paid to identifying the cause of secondary hypertension, as most causes of secondary hypertension are potentially correctable. Over 90% of people with severe renal disease have hypertension.15 This patient had resistant hypertension, pointing to secondary hypertension with BP in the range of 160/100–230/140 mmHg. Because he had a moon-face appearance, secondary work-up for hypertension should have been performed, but it was not done due to resource limitations. Studies and experts have emphasised that patients with CKD who develop hypertension and are treated with more than four antihypertensive medications without improvement should be referred to a nephrologist.16 For this patient, more than four antihypertensive medications were used, but his BP remained high from the day of admission until the day of discharge. No referral to a nephrologist was made because of resource limitations and poor socioeconomic status. Thus, comprehensive CKD management was interrupted.
Regarding the management of patients with CKD, it is good medical practice to institute RRT before the onset of uremic syndrome. However, this fact is profoundly and ethically violated due to the non-accessibility and non-affordability of RRT, especially in resource-constrained healthcare settings. RRT may be accomplished via kidney transplantation or dialysis. Kidney transplantation is the most sustainable and preferable approach. Dialysis can be accomplished either by haemodialysis or peritoneal dialysis. In 2019, the International Society of Nephrology (ISN) developed the framework to guide the establishment or expansion of chronic dialysis programmes in low-resource settings as part of the ISN’s work with the World Health Organization (WHO).34 However, these services are still not comprehensively and equitably available for the patients who need them, especially in developing nations.35-37 Further actions are urgently needed to ensure the proper management of patients with CKD as one of their fundamental human rights. For CKD patients, the criteria for dialysis include GFR <10 mL/min/1.73 m2, creatinine clearance <15 mL/min, chromium >1,000 mmol/L (>11.3 mg/dL), urea >30 mmol/L (>83 mg/dL), albumin <35 g/L (<3.5 g/dL]), uraemic symptoms, and any acute indications.29 This patient had all these; thus, he qualified for emergency RRT. However, because of resource-limited healthcare settings, RRT was not provided to him. A renal transplant would have been the most profitable therapy for him. The availability of dialysis services would also increase his survival chances. Peritoneal dialysis would be the preferred dialysis approach because of the resource-limited healthcare setting,38 and because peritoneal dialysis has numerous advantages over haemodialysis. Among those advantages are the short time spent in dialysis units, the fact it does not necessitate strict dietary limitations compared to haemodialysis, the rehabilitation rates are better than those detected in haemodialysis, it gives the opportunity to return to full-time employment for most patients, and it also ensures that residual renal function is maintained for a longer period (e.g., 1–2 years), hence improving morbidity and mortality.6,38,39 He was discharged with fairly good improvement, but predictably, in the near future, he will be admitted with similar complaints. In fact, his prognosis will likely remain very poor without RRT.
CONCLUSION
The main purpose of this manuscript has been to highlight CKD as one of the most serious non-communicable diseases that is unfortunately still neglected in resource-limited healthcare settings, using the case of a 28-year-old male with CKD who developed ESRD. He qualified for RRT; however, RRT was not provided to him due to its non-accessibility, non-affordability, and resource-limited healthcare settings. Renal diseases, including CKD, are commonly encountered in clinical practice in both developed and developing countries. The lack of RRT marks the mistreatment of patients with renal diseases by the global healthcare system. The author calls for designing new strategies that aim to ensure equitable accessibility and affordability for RRT globally. One of these strategies should be to deal with companies that manufacture and sell the machines, such as dialysis machines and other equipment and substances used for RRT, to ensure cost-effectiveness and their availability in developing countries where resource-limited healthcare is profoundly common.
Ethical Consideration
After explaining the purpose and potential advantages of publishing this case to the patient and his parents, they gave their consent. They received guarantees that neither their names nor the publication’s intended audience would be revealed.







