Abstract
Background: Central line-associated bloodstream infection (CLABSI) is a serious infection typically increasing morbidity and mortality in patients with chronic kidney disease (CKD). It can be prevented through proper insertion techniques and management of the central line (CL). However, the first step in reducing the CLABSI rate is to define the extent of the problem through proper surveillance. This study aimed to determine the frequency of CLABSI in patients with CKD at a specialised renal care centre.
Methods: The authors conducted a retrospective observational study to determine the frequency of CLABSI in patients with CKD between November 2021–September 2022 at their institute. They included all patients with CLs registered at their institute. Primary CLABSI was defined as CLABSI attributable to their hospital, while secondary CLABSI was defined as those not attributed to their hospital.
Results: Fifty-nine incidences of CLABSI were identified in a total of 310 patients with CL and 1,413 CL days, giving a total of 42 CLABSI incidences per 1,000 CL days. Primary CLABSI was more common (n=36 [61%]) than secondary CLABSI (n=23 [39%]). Most of the patients recovered (53 [89.8%]); however, four (6.9%) patients expired. Most of the patients who recovered had permanent vascular access (n=32 [60.4%]), internal jugular placement (n=44 [83%]), and primary CLABSI (n=33 [62.3%]), although the p-values were non-significant.
Conclusion: Strict implementation of CLABSI prevention bundles for line insertion and its maintenance and regular surveillance using laboratory confirmed cases is needed to reduce the rates of CLABSI.
Key Points
1. Central line bloodstream infection (CLABSI) is a leading cause of high morbidity and mortality in patients with chronic kidney disease (CKD) who are dialysis dependent.2. This was a retrospective observational study to determine the frequency of CLABSI in patients with CKD at a renal care centre.
3. Appropriate surveillance of the disease, followed by strict aseptic measures during central line insertion and maintenance, are the key steps to reduce CLABSI in patients with CKD.
BACKGROUND
Infection leads to an increased rate and duration of hospitalisation, increased treatment costs, and high morbidity and mortality in patients with chronic kidney disease (CKD) with end-stage renal disease. Its major risk factor in patients on chronic haemodialysis (HD) is vascular access, especially central line (CL),1-7 which leads to central line-associated bloodstream infection (CLABSI), resulting in life-threatening complications.8-10
Appropriate infection prevention and control (IPC) measures taken at the time of insertion and maintenance can prevent CLABSI.11
A focus on education and strict adherence to IPC measures is associated with reducing its rate.12,13 However, the first step in reducing the CLABSI rate is to define the extent of the problem through proper surveillance.14,15 There is a high burden of CLABSI in patients with CKD, as evident by the past data.1,7,16,17 This study aimed to determine the frequency of CLABSI in patients with CKD at a specialised renal care centre, to add to the previous existing literature from the authors’ country.
METHODS
The authors conducted a retrospective observational study to determine the frequency of CLABSI in patients with CKD between November 2021–September 2022 at their institute, which is a not-for-profit specialised hospital providing care to patients suffering from kidney disease.
An exemption was obtained from the institute’s ethical review committee (ref. no. 152-IDC-102022(EXEMPTION).
Using the Centers for Disease Control and Prevention (CDC) surveillance definition,14,18 CLABSI was defined as a laboratory-confirmed bloodstream infection (LCBI) that was not secondary to an infection at another body site, where an eligible bloodstream infection organism was identified and a CL had been in place for more than two consecutive calendar days on the LCBI date of an event or the day before.
CL was defined as an intravascular catheter that terminated at or close to the heart, or in one of the great vessels, used for dialysis, infusion, withdrawal of blood, or haemodynamic monitoring.
The authors included all patients with CLs registered at their institute; these included patients admitted with them, those routinely receiving dialysis at the institute, and those who presented to the emergency room. They excluded non-registered patients and registered patients without any CL. CL days were assessed to determine if an LCBI was a CLABSI. The count of CLs was recorded in the monthly IPC hospital acquired infections data.
The authors modified the CDC criteria and also identified CLABSI not attributed to their institute. Primary CLABSI was defined as CLABSI attributable to them, while secondary CLABSI was defined as those not attributed to their hospital.
STATISTICAL ANALYSIS
The data were analysed on SPSS version 26 (IBM, Armonk, New York, USA). Continuous variables were evaluated using mean with standard deviation, while categorical data were evaluated using frequency with percentage. The ꭓ2 test observed association among variables. A p-value of ≤0.05 was considered statistically significant.
RESULTS
Fifty-nine incidences of CLABSI were identified in a total of 310 patients with CL and 1,413 CL days, giving a total of 42 CLABSI incidences per 1,000 CL days. Primary CLABSI was more common (n=36 [61%]) than secondary CLABSI (n=23 [39%]), and there were more male cases (36 [61%]) than female ones (n=23 [39%]). The mean age was 53±17.8 years, and the median age was 57, with an interquartile range of 27 years. Most patients had permanent vascular access (n=36 [61%]). The internal jugular was the major site of choice (n=49 [83.1%]; Table 1). Most CLs (n=37 [63%]) were inserted using ultrasound guidance. Most of the patients recovered (53 [89.8%]); however, four (6.9%) patients expired (Figure 1). Most of the patients who recovered had permanent vascular access (n=32 [60.4%]), internal jugular placement (n=44 [83%]), and primary CLABSI (n=33 [62.3%]), although the p-values were non-significant (Table 2).
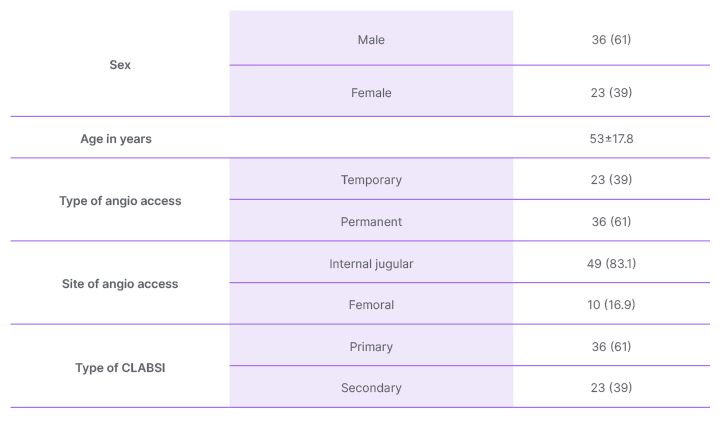
Table 1: Baseline characteristics of the patients with central line-associated bloodstream infection
(n [%] /mean±standard deviation).
CLABSI: central line-associated bloodstream infection.
Staphylococcus aureus; Enterobacterales, including Enterobacter spp. (such as Enterobacter cloacae and other species), Klebsiella spp., Escherichia Coli, and Citrobacter freundii; Candida spp., including Candida tropicalis, Candida parapsilosis, and other Candida spp.; Enterococcus spp.; Acinetobacter spp.; Pseudomonas aeruginosa; Stenotrophomonas maltophilia; and other Pseudomonas spp., including Ralstonia pickettii, Shewanella putrefaciens, and Chryseobacterium spp., were identified in decreasing order of
their occurrence (Figure 2).
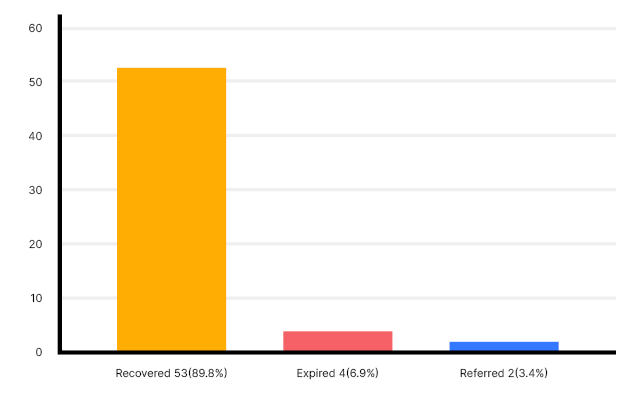
Figure 1: Outcome of the patients.
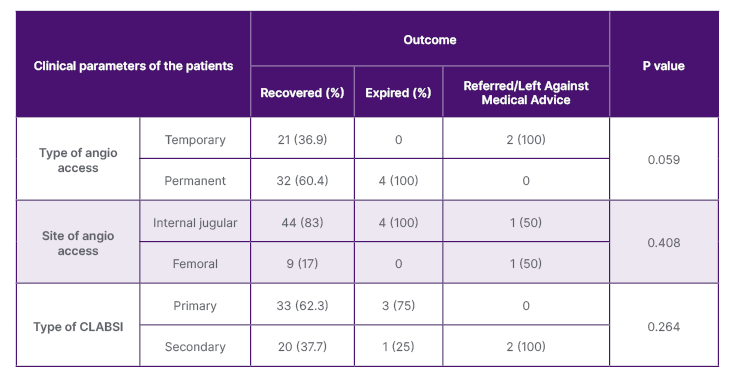
Table 2: Association of clinical characteristics of the patients with the outcome.
CLABSI: central line-associated bloodstream infection.
DISCUSSION
The authors’ institute is a specialised hospital providing care to patients with CKD. The majority of these patients receive HD, for which temporary or permanent vascular access is required.16 The authors aim for an arteriovenous fistula (AVF) or arteriovenous graft for better clinical outcomes and lower infection rate;19,20 however, temporary access is needed in cases where permanent access is either not available or not ready to be used. It is achieved with a CL, which can be permanent (tunnelled/implanted), or temporary (non-tunnelled), but chances of infection are generally higher in these.8 In a previous study from the authors’ institute, Qureshi et al.16 concluded that out of a total of 429 non-tunnelled CLs, 50 (17%) were removed due to CLABSI. Similar to the authors’ data, S. aureus was the most common organism responsible for CLABSI.16 Another previous study from the authors’ institute also shows a high burden of S. aureus CLABSI in patients with CKD.7 This is consistent with the previous data from the authors’ country, which shows a high burden of S. aureus CLABSI in patients with CKD.21 However, this differs from the National Healthcare Safety Network (NHSN) data from January 2006–October 2007, where an estimated 250,000 bloodstream infections occur annually, and most are related to the presence of intravascular devices. The order of selected pathogens associated with CLABSI was as follows: 34.1% coagulase-negative Staphylococcus spp.; 16.0% Enterococcus spp.; and 9.9% S. aureus; followed by Gram-negatives with 5.8% Klebsiella spp., 3.9% Enterobacter spp., 3.1% Pseudomonas spp., 2.7% E. coli, and 2.2% Acinetobacter spp.; Candida species (11.8%); and others (10.5%).22
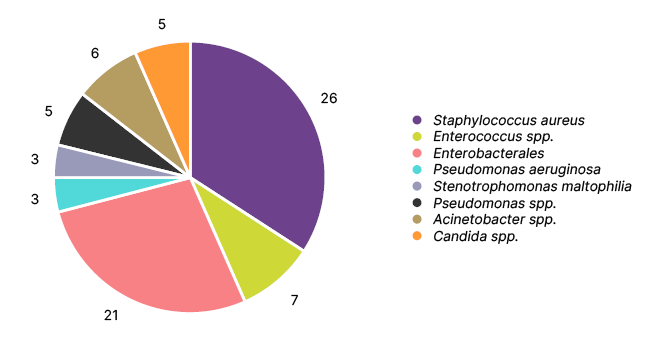
Figure 2: Frequency of organisms isolated.
In the authors’ study, out of a total of 59 incidences of CLABSI, 36 (61%) were tunnelled (permanent) catheters, and 23 (39%) were non-tunnelled ones. The internal jugular vein was used in 50 (85%) cases, while the femoral vein was used in nine (15%) cases.
These findings contrast with Zanoni et al.,8 who concluded that as compared to tunnelled jugular CL, a higher CLABSI rate was associated with non-tunnelled jugular and non-tunnelled femoral CL use. They also concluded that non-tunnelled and femoral CL were associated with higher CLABSI rates.8 The possible reason for more internal jugular lines can be the fact that in the authors’ hospital, most lines are inserted into these, as also supported by the findings of Qureshi et al.16 Likewise, the practice of promoting tunnelled catheters instead of non-tunnelled ones at the authors’ institute could be a reason for more CLABSI cases in tunnelled ones.
In an Irish cohort, while determining the outcomes of access-related bloodstream infections in patients on HD, Mohamed et al.3 concluded that access-related bloodstream infection was more associated with CLs than AVF in HD, and AVF preference over CL should be emphasised.
There was a high rate of CLABSI. Proper surveillance of CLABSI is essential for determining the frequency of its incidence, and thus for preventive measures. The majority of CLABSI incidences were attributable to the authors’ centre. However, the authors also identified cases where the line was inserted/maintained at other institutes, and these were considered as secondary CLABSI.
In addition to adding to the existing local data of CLABSI, the authors’ data emphasise that IPC practices, including preventive bundles during line insertion and maintenance, need to be strengthened, together with proper surveillance of rates of infection. Major areas of emphasis include strict adherence to aseptic measures, i.e., CLABSI prevention insertion and maintenance bundles, especially focusing on proper sterile barrier precautions, together with skin antisepsis with >0.5% chlorhexidine preparations. Focus should also lie on pre-procedure patient shower or bed sponge with chlorhexidine. Previous studies support that adequate surveillance followed by strict adherence to CLABSI prevention insertion and maintenance bundles, which include, but are not limited to, hand hygiene, continuous training of healthcare providers, chlorhexidine bath and skin antisepsis, proper sterile barrier precautions, use of ultrasound guidance to prevent multiple pricks, chlorhexidine dressings, minocycline/rifampin catheters, and removal of the line as soon as possible, lead to a reduction in the rate of CLABSIs.13,23 The authors are emphasising on these prevention bundles, with proper hand hygiene as the key to all IPC measures. Regular teaching, on the spot reminders, and frequent and surprise audits are being practised to reduce the CLABSI rates.
A small sample size is a major limitation of the authors’ study. Secondly, many samples yielded multiple organisms, though they fulfilled the CDC criteria for CLABSI, and thus were included in the study. Still, these data help in monitoring the frequency of CLABSI at the institute through surveillance and focusing on the education of healthcare providers in aseptic line insertion and maintenance bundles.
CONCLUSION
Strict implementation of CLABSI prevention bundles for line insertion and its maintenance and regular surveillance using laboratory confirmed cases is needed to reduce the rates of CLABSI.







