Achieving equal and accurate representation in clinical trials has long been a challenge for the pharmaceutical industry. We have made great progress towards the goal of patient inclusivity, but what more can be done to ensure that all patient groups are adequately represented and how can digital help to achieve this?
Words by Michaila Byrne
In this day and age, when you walk into a shop, you can rightly expect to browse through rails draped with clothes designed for all genders, sizes, ages, and shapes. As fashion and culture have become more inclusive, so has medical research, and in particular, clinical trial recruitment. Connectivity has improved on a mass scale, and as a result the pharmaceutical industry’s access to more patient populations has improved. How can pharma build on its past progress to identify current gaps in clinical trial recruitment and utilise digital platforms in their pursuit to make clinical trials as representative and diverse as possible?
“Improved recruitment methodologies lead to a better selection of patients into the trial, which has a positive impact on trial quality and effectiveness,” explains Zakia By, Vice President, Clinical, Medical and Regulatory, APAC, Novo Nordisk. A lack of representation in clinical trials is not simply an ethical concern, but a medical one too. Theresa Rohr-Kirchgraber, MD, Professor of Clinical Medicine and Paediatrics, Indiana University School of Medicine, reflects on past errors that have occurred as a result of underrepresentation in clinical trials: “When Ambien first came out, the majority of clinical trials were done in men so there were multiple situations where patients were having problems and difficulty metabolising the medication. It was recommended as a 10mg dose. Now, if you are a woman, the recommendation is 5mg.”
Thankfully, recruitment channels for clinical trials have moved from papers and radio, to online and social media, which has had a profound impact on the selection of recruits and ultimately the range of patients the industry can evenly serve. Camilla Krogh Lauritzen, Chief Patient Officer, LEO Pharma, views this evolution as: “Very much reflective of the general transition of paternalistic medicine to participatory medicine; the patient is now considered an important part of the solution in general, including a partner in both the design and delivery of clinical trials.”
Improved recruitment methodologies lead to a better selection of patients into the trial
Clinical trials have marketing budgets to ultimately onboard larger and more appropriate numbers of patients and digital channels have the potential to facilitate this goal more than ever before. “Digital advertising makes patient outreach and the spread of trial information much simpler and progressive compared with conducting patient camps, searching physical patient databases and outpatient department cards, and letters to patients,” says By. She reiterates that: “Continuous joint efforts are needed at industry level to endorse the new digitisation era in clinical trials in order to make it a success that benefits all involved parties.”
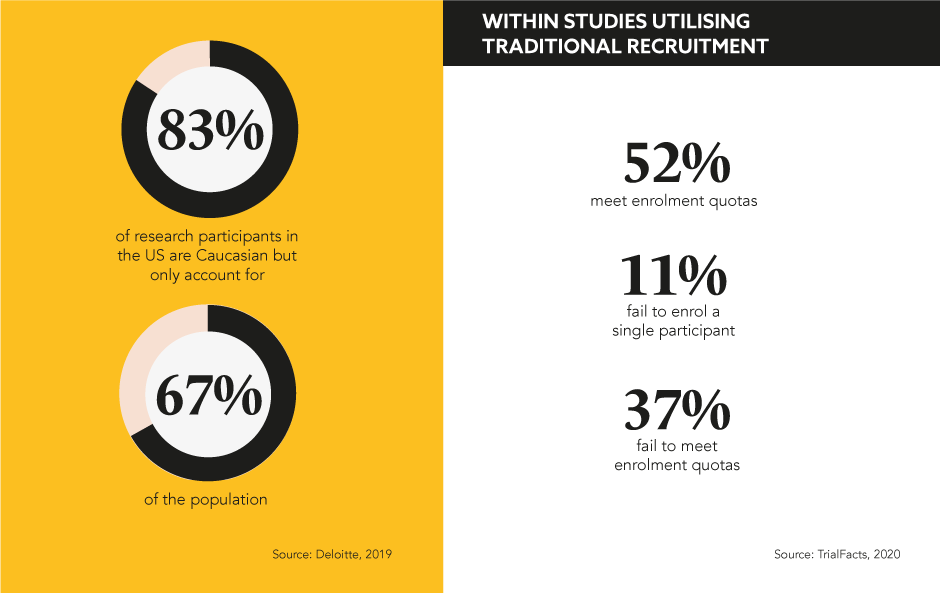
The industry has made great progress and advancements in this realm, but what barriers in clinical trial recruitment remain that could be preventing 100% patient inclusivity?
Many make the argument that historical, language, and even trust barriers can prevent people from participating in clinical trials, and evidence indicates that research participation is lower for ethnic minorities and underrepresented groups like the elderly or pregnant women. To actively counteract this gap in medical research, marketing teams need to target the right communities and build a presence, online or offline, to recruit patients. To achieve this, Krogh Lauritzen advises pharma to partner with more patient organisations: “I am convinced that in the future, patient communities will play an even bigger role than they do today in the recruitment side of clinical trials… they are the experts in the disease at question and know the patient population better than anyone.” Evidently, regular and active engagement with patient populations online is vital, otherwise, clinical trial outcomes are much more likely to be unrepresentative of patient outcomes in the real world.
Rohr-Kirchgraber identifies that much more needs to be done in the area of targeted recruitment, reminding us that: “Even though in 2010 we had mandates to increase the number of women in clinical trials; in 2018, 80% of the participants in clinical trials were men, even when factoring in disease specifics.”
For medicine to move forward and for the industry to fully eradicate medical research bias, marketing teams must harness the momentum we are seeing towards increased inclusion and channel it into clinical trial recruitment. More innovative methodologies could ensure that any potential gaps in the data are filled at these early, crucial stages and that our drugs are tailored to all patient populations, rather than just a select few.