Meeting Summary
Prostate cancer has traditionally been staged through the use of conventional imaging techniques such as bone scintigraphy, CT, and MRI. However, the introduction of more sensitive techniques, such as prostate-specific membrane antigen (PSMA) imaging, has allowed previously undetectable metastases to be identified, thereby enabling more accurate staging of the disease and greater refinement in management strategies.
This article summarises a symposium delivered on 3rd July 2022 at the 37th European Association of Urology (EAU) Annual Congress in Amsterdam, the Netherlands, where speakers from three different specialties raised important questions in prostate cancer imaging. Stefano Fanti, Professor of Diagnostic Imaging/Director from S. Orsola Policlinic Hospital, Bologna, Italy, asked: “What is PSMA all about?”, and Jochen Walz, Professor of Urology and Head, Department of Urology at the Institut Paoli-Calmettes Cancer Centre, Marseille, France, wondered: “When does PSMA help me?” Alicia Morgans, Genitourinary Medical Oncologist/Medical Director at the Dana-Faber Cancer Institute, USA, then offered an overview of the future of prostate cancer management. The session concluded with the presentation of three cases of patients with different stages of prostate cancer, all of which illustrated the transformative benefit of PSMA imaging in accurately staging patients and directing subsequent treatment options.
Introduction
Imaging has a pivotal role in the staging of primary prostate cancer, as well as in determining optimal management strategies.1 Conventional imaging modalities used in prostate cancer have traditionally included CT, MRI, and 99mTc-labelled bisphosphonate bone scintigraphy.2 Although widely available, these modalities have significant limitations, including poor sensitivity and specificity for metastatic disease.3 A desire to find a better diagnostic imaging technique for prostate cancer led to a focus on prostate-specific membrane antigen (PSMA), which is highly overexpressed in most prostate carcinoma cells.1 Since its introduction in 2011, PET imaging with agents targeting PSMA has demonstrated value in the staging of recurrent prostate cancer, and has increasingly been adopted in clinical practice.1 The most widely studied PSMA radioligand is 68Ga-labelled PSMA-11 (68Ga-PSMA-11 [ABX Advanced Biochemical Compounds GmbH, Radeberg, Germany]).2,4
In the Telix-sponsored symposium, ‘Improvements in prostate cancer management: focus on imaging and treatment’, delivered on 3rd July 2022 at the 37th EAU Annual Congress in Amsterdam, the Netherlands, speakers from three different specialties invited delegates to ‘get on board the PSMA train’ to optimise the imaging and treatment of prostate cancer, and help improve outcomes for patients.
What Is Prostate-Specific Membrane Antigen All About?
Stefano Fanti
Fanti opened the symposium by asking: ‘What is PSMA all about?’ He explained that PSMA is a unique membrane-bound glycoprotein that is overexpressed in prostate cancer as well as the neovasculature of most solid tumours, but is not expressed in the vasculature of normal tissues.5 Fanti described how this overexpression makes PSMA an important marker in solid tumours, including (but not only) prostate cancer.6 PSMA expression is higher in high-grade and castration-resistant prostate cancer (CRPC) than in less advanced forms of the disease.7
The small molecule inhibitor of PSMA, 68Ga-PSMA-11, binds with high affinity to the enzymatic pocket of PSMA, where it remains trapped within the prostate cancer cell.8 Use of the PSMA tracer with PET/CT allows functional imaging of the body to identify localised ‘hotspots’ of prostate cancer.
Performance
Fanti explained that the performance of an imaging technique may be described in terms of its sensitivity and specificity. Data from one of the first large meta-analyses of 68Ga-PSMA PET showed that this technique was effective in detecting metastases in patients with biochemical recurrence of prostate cancer, and had favourable per-node sensitivity (75%) and specificity (99%).4 The value of PSMA PET scanning for disease staging is now reflected in guidelines on the evidence-based management of prostate cancer.9
Fanti next compared 68Ga-PSMA-11 with other tracers for the imaging of patients with prostate cancer. One study of patients with biochemical recurrence of prostate cancer and low prostate-specific antigen (PSA) levels (<2 ng/mL) after radical prostatectomy who underwent both 18F-fluciclovine and PSMA PET/CT scans within 15 days of each other demonstrated the clear superiority of 68Ga-PSMA-11 in terms of the biochemical recurrence detection rate.10 This study was unusual in that the same patients were imaged with both techniques, and supports the positioning of PSMA as the PET tracer of choice in this setting. Data from a systematic review of 98 individual studies examining the role of imaging in early recurrent prostate cancer also demonstrated a higher detection rate for 68Ga-PSMA-11-based imaging compared with any other imaging modality, including CT or MRI.11
Reliability
Fanti noted that although PSMA PET is the most accurate and sensitive method for detecting the biochemical recurrence of prostate cancer, issues remain in terms of reliability. He explained that if a patient has recently been treated with radiotherapy or brachytherapy, inflammatory changes may induce local uptake of the tracer that could be misinterpreted as metastatic disease. An experienced physician would understand that a solitary faint signal in a rib does not necessarily indicate pathological changes.12 Further, any risk of misinterpretation could be mitigated through a judicious choice of PSMA tracer, with the onus being on the nuclear medicine physician to select the most appropriate tracer for a given situation. For example, data suggest that in patients with biochemical recurrence of prostate cancer, 18F-PSMA-1007 (ABX Advanced Biochemical Compounds GmbH, Radeberg, Germany) may be suitable for the detection of locoregional lesions near the urinary tract, whereas 68Ga-PSMA-11 may be a better option for the identification of distant metastases such as those in bone.13 Another study showed that 68Ga-PSMA-11 PET/CT gave fewer false positives than 18F-PSMA-1007 PET/CT in patients with biochemical recurrence of prostate cancer after radical prostatectomy (Figure 1).14
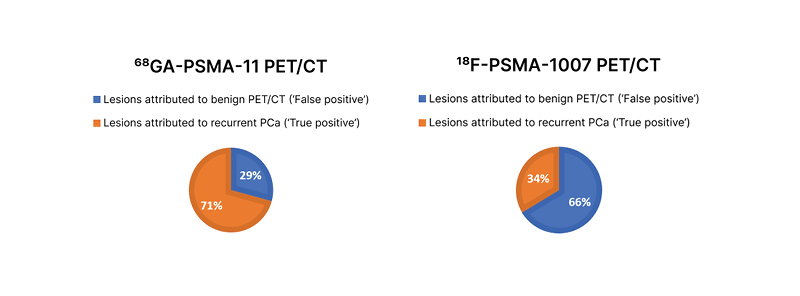
Figure 1: Distribution of suggestive and benign lesions for all PSMA-ligand–positive lesions on 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT.
Adapted from Rauscher et al.14
PCa: prostate cancer; PSMA: prostate-specific membrane antigen.
Fanti commented that “if the [aim] in biochemical recurrence is to find possible sites of metastases at the bone level, it is clear I would favour 68Ga-PSMA-11 over 18F-PSMA-1007.”
Fanti concluded by noting that PSMA PET has been a fantastic success in prostate cancer imaging, but that the optimal use of this technique is all about the choice of tracer. He advised that tracer selection should not be made on the basis of cost or availability, but should be based on the performance, robustness, and reliability of the tracer in question.
When Does Prostate-Specific Membrane Antigen Help Me?
Jochen Walz
In order to answer the question, “when does PSMA help me?”, Walz began by setting out the different clinical scenarios in which PSMA imaging can be used, including initial staging after diagnosis, staging in patients with persistent PSA after radical prostatectomy, and staging at the time of biochemical recurrence or disease progression.
Initial Staging
Walz first described the ProPSMA study in which 68Ga-PSMA-11 PET/CT outperformed conventional imaging in males with biopsy-proven prostate cancer and high-risk features in terms of accuracy, specificity, and sensitivity, with particular sensitivity in the lymph nodes.15
Walz next introduced the concept of ‘stage migration’, in which a more sensitive imaging tool allows the true position of a patient along the prostate cancer spectrum to be determined (Figure 2).16,17 This approach may help to identify the optimal balance between local and systemic therapies and thereby improve patient outcomes. In the absence of available data to understand how this information may be used in practice to change disease management, Walz explained that patients currently need to rely on expert opinion to direct treatment based on PSMA PET.9,18
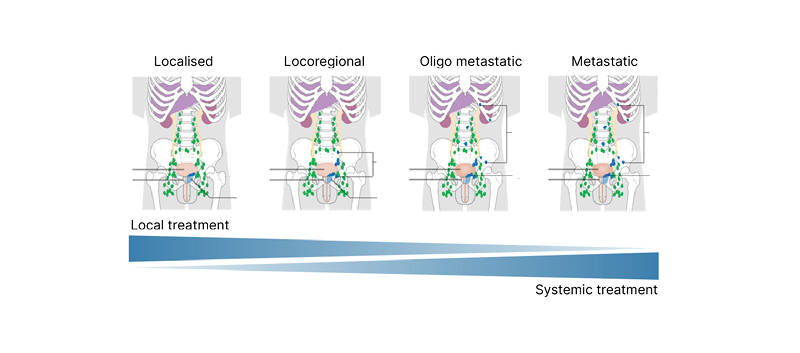
Figure 2: Spectrum of prostate cancer.
Staging in patients with persistent PSA after radical prostatectomy.
Adapted from two images16,17 by Cancer Research UK (2015) under the terms of the Creative Commons Attribution -ShareAlike 4.0 International (CC BY-SA 4.0) licence, via Wikimedia Commons.
Walz described how treatment options vary according to the region of the body in which the prostate cancer persists. For example, local persistence in the prostatic bed and regional persistence in the pelvic lymph nodes are both potentially curable with regional salvage treatment, while distant or systemic persistence in the lymph nodes or bone requires systemic treatment.
Patients with PSA persistence have a higher risk of disease progression at 15 years compared with those with an undetectable PSA.19 PSMA imaging in patients with persistent PSA often detects pelvic lymph node metastases outside the standard field, in the obturator, presacral, or mesorectal nodes.20,21 PSMA imaging, therefore, helps to stage these high-risk patients and direct whether local or systemic treatment is required. Even in patients with early PSA persistence (0.1–0.2 ng/mL), PSMA imaging can help locate disease.21 Use of PSMA imaging in cases of persistent PSA after surgery is also recommended by the guidelines,9 although there is currently uncertainty regarding the best treatment approach when PSMA PET or CT reveals metastatic disease.
Staging at Biochemical Recurrence or Progression of Prostate Cancer
Walz explained that an early understanding of whether rising PSA is due to local recurrence, locoregional recurrence, or systemic recurrence is highly beneficial, as it allows early intervention with the most appropriate treatment. PSMA imaging is able to detect lesions even in patients with low PSA levels (>0.2 ng/mL).4
Emerging data suggest that new imaging approaches are improving the treatment of patients and guiding the use of post-prostatectomy salvage radiotherapy,22 salvage lymph node dissection,23 and metastasis-directed therapy.24
Walz noted that it is not completely clear how clinicians should act on the information provided by PSMA imaging in this setting, and again need to rely on expert recommendations until further data become available.18 It is important, Walz concluded, that “care should be taken to avoid unproven treatment decisions that may result in under-treatment (or over-treatment), and finally harm to patients.”25
The Future of Prostate Cancer Management
Alicia Morgans
Morgans concluded the formal presentations by identifying forward-thinking clinical applications of advanced imaging that might provide the greatest benefit to patients with prostate cancer.
Defining New Disease States
Echoing an earlier point from Walz, Morgans noted that it is critical to treat patients with the right therapies based on their stage migration, and that the use of PET imaging for high-risk localised disease allows the identification of metastatic lymph nodes that would not be visible on conventional CT or bone scans. Data from the STAMPEDE trial26 show that intensification of androgen deprivation therapy (ADT) with abiraterone improves metastasis-free survival and overall survival in patients with clinically lymph node-positive disease or very high-risk localised disease.27
Morgans also showed data from the ORIOLE trial,28 which demonstrated that stereotactic ablative radiation of all lesions visible on PSMA imaging may help to improve outcomes in patients with biochemical recurrence.29 Finally, they described how PSMA imaging identifies previously undetectable metastases lesions in many patients (>98%) with non-metastatic CRPC based on conventional imaging.30 Thus, PSMA imaging may help to guide a more accurate determination of stage migration, refine treatment options in the non-metastatic CRPC setting, and further improve outcomes, yet Morgans emphasised patients should still receive the intensified systemic treatment to prolong metastasis-free survival and overall survival.30
Selection for Treatment
Morgans next described the important role of PSMA-11 in identifying candidates for treatment with 177Lu-PSMA-617 (Advanced Accelerator Applications, Saint-Genis-Pouilly, France) a radioligand recently approved by the U.S. Food & Drug Administration (FDA) for the management of metastatic CRPC. The VISION trial31 showed that the addition of 177Lu-PSMA-617 to standard therapy improved progression-free survival and overall survival in patients with metastatic castration-resistant cancer detectable by PSMA-11 imaging.32 Morgans noted that effective therapies of this type are only made possible with the availability of suitable imaging agents such as PSMA-11.
Future Opportunities in Radioligand Therapy
Morgans concluded her presentation by introducing the novel radioligand, TLX591 (Telix Pharmaceuticals, Melbourne, Australia), which is a monoclonal antibody construct that binds with high affinity to PSMA with limited off-target effects. TLX591 has been shown to have clinical efficacy and an acceptable safety profile as a potential alternative to PSMA-directed small molecules (Figure 3).33,34 Morgans suggested that this innovative therapeutic approach may offer new opportunities for patients with prostate cancer.
Figure 3: Prostate-specific membrane antigen radiolabelling patient experience.
Data courtesy of Scott Tagawa.
PSMA: prostate-specific membrane antigen.
Patient Cases Discussion and Roundtable
Stefano Fanti, Jochen Walz, and Alicia Morgans
Following conclusion of the formal presentations, the panel members discussed three real-world case studies to illustrate the use of PSMA imaging in the settings described.
Case 1: Biochemical Recurrence (Hormone Sensitive)
Morgans presented the case of a 69-year-old male with a history of prostate cancer who was treated with a prostatectomy in July 2016. At the time of prostatectomy, he had a PSA of 7.2 ng/mL, and pT3a pN0 M0 R1 (positive right apical margin) prostate adenocarcinoma with a Gleason score of 4+4. Their PSA was undetectable within 6 weeks after surgery. They underwent adjuvant radiotherapy in September 2016, and then his PSA was monitored by a urologist. Their past medical history included hypertension controlled with hydrochlorothiazide, while their grandfather had had prostate cancer and their father had had heart disease. In April 2021, his PSA had risen to 0.3 ng/mL from 0.2 ng/mL in March 2021.
Walz noted that because this patient had already received adjuvant radiotherapy, there was no urgency to proceed with salvage therapy. Walz indicated that more information about PSA kinetics would be valuable. Walz also noted that the substantial length of time between the initial disease and the subsequent increase in PSA suggested that the disease may not be aggressive in nature.
The patient’s PSA increased further to 0.5 ng/mL in May 2021 and then to 1.2 ng/mL in August 2021. At this point, the patient underwent 68Ga-PSMA PET imaging to gain additional information about the status of his recurrent disease.
PSMA imaging revealed intense uptake in the kidney, liver, and spleen as well as hotspots in the lower pelvis. However, Fanti explained that the sagittal view showed only superficial uptake in the skin in this area, most likely indicating tracer contamination rather than pathological changes. However, Fanti also highlighted two other focal hotspots corresponding to lymph nodes, which he would report as suspicious. He noted that such a relatively intense uptake, focally localised at the lymph node, would be very unlikely to be a false positive.
Walz agreed that this patient is likely to have a locoregional relapse, and would be a candidate for radiotherapy or a regional salvage treatment depending on what adjuvant therapy had been given previously. Walz concluded that several treatment options would be available for this patient and that these would in practice be discussed and agreed within a multi-disciplinary team.
Case 2: Staging High-Risk Localised Disease
In the second case, a 67-year-old male with a history of osteoarthritis was found to have a left-sided nodule following a digital rectal examination as part of his annual physical examination. Their PSA at this time was 13.8 ng/mL, and the patient felt well except for recent incidents of nocturia. The patient underwent a prostate biopsy that demonstrated Gleason 4+5=9 (Grade group 5) prostate adenocarcinoma in three of six cores on the left side and Gleason 3+4=7 (Grade group 2) prostate adenocarcinoma in two of six cores on the right side. The patient presented for further management of their high-risk localised prostate cancer and underwent a bone scan.
Fanti observed that the bone scan was negative as expected, given the low sensitivity of the modality, and observed that PSMA imaging rather than a bone scan would now routinely be performed in these high-risk patients. Walz agreed that as this is a true high-risk patient with high volume disease, determination of disease stage would be required using the most sensitive imaging technique available; ideally PSMA.
Fanti interpreted the PSMA PET scan by observing several hotspots, including two in the thorax corresponding to lymph nodes. He noted that in some patients, uptake in the lung could be due to permanent inflammation of the lymph nodes, possibly caused by smoking. However, Walz determined that the intensity of the uptake in this case, together with other PSMA PET findings, suggested metastatic disease. Morgans added that the CT scan for this patient was negative, confirming that a negative CT scan should not undermine the findings from a more sensitive PSMA scan.
Walz noted that additional scanning might be necessary to confirm that this patient requires systemic treatment. Walz would also request a biopsy of the lymph nodes in the chest. Fanti noted that in similar cases, a biopsy has frequently confirmed prostate cancer metastasis. This together with the presence of bone metastases detected by PSMA imaging makes it less likely that a biopsy would be performed. Walz agreed that a biopsy would be most important if the lung lesions were the only lesions that would make a difference to the treatment approach.
Case 3: Non-metastatic Castration-resistant Prostate Cancer
Walz presented the final case of a 64-year-old male with a history of prostate cancer (diagnosed in November 2018) who was treated with prostatectomy in January 2019. At the time of prostatectomy, they had a PSA of 11.6 ng/mL and pT3b pN0 M0 Gleason 4+4 prostate adenocarcinoma. The patient’s PSA was 0.2 ng/mL 6 weeks after surgery, and they underwent salvage radiotherapy with ADT for 6 months in March 2019. While receiving ADT, his PSA increased from 1.6 ng/mL in October 2019 to 1.9 ng/mL in January 2020.
Morgans commented that these findings are suggestive of castration resistance and that they would request measurement of testosterone levels. They would also request a PSMA PET but feared that this patient was showing signs of aggressive disease.
The patient’s PSA continued to rise from 2.00 ng/mL in February 2020 to 3.81 ng/mL in October 2020, with a doubling time of 8.6 months, and his testosterone level was <20 ng/dL (castrate level). A CT scan of the abdomen and pelvis with intravenous contrast and a technetium bone scan were both negative. Fanti noted multiple hotspots on the PSMA PET scan, including several bone and nodal lesions, which would be reported as suspect.
Morgans observed that ‘non-metastatic’ CRPC is only really non-metastatic according to conventional imaging, while newer imaging techniques typically reveal these patients to have metastases in reality. Morgans said that they would treat this patient systemically with an androgen receptor antagonist in addition to ADT, but not with additional radiotherapy given the number and location of the lesions. They noted that the patient outcome may have been improved if it had been evaluated at an earlier, hormone-sensitive stage.
Conclusion
The presentations in this symposium demonstrated the effectiveness of 68Ga-PSMA-11 PET/CT in the detection of prostate cancer tumours compared with other imaging modalities, and highlighted the clinical settings in which this technique adds value. The speakers illustrated how 68Ga-PSMA-11 PET scans can inform and potentially improve patient management and survival. It was also noted that this modality may be used to identify candidates for therapies such as PSMA-targeted radioligand therapies. Finally, the speakers demonstrated how novel radioligand therapeutics, including monoclonal antibodies, may offer new opportunities in the treatment of patients with prostate cancer. The symposium closed with the presentation of three case studies, which demonstrated the clinical utility of PSMA imaging in clinical practice, illustrating the value of this technique and providing insights into how PSMA PET imaging is having a transformative effect on the management of prostate cancer.








