Abstract
Polyacrylamide hydrogel bulking agent (Bulkamid® [Axonics, Irvine, California, USA]) injection is used as a minimally invasive treatment for stress and mixed urinary incontinence in females. Several studies have demonstrated the short- and medium-term efficacy and safety of Bulkamid. However, there are limited data available on the long-term safety of this procedure. The authors report an unexpected and late complication associated with Bulkamid periurethral injection.
An 80-year-old female, who had undergone Bulkamid periurethral injection for stress urinary incontinence 10 years previously, was referred to the authors’ clinic with recurrent lower urinary tract symptoms and dysuria. Investigations with ultrasound and cystoscopy confirmed a single 2 cm bladder stone adhered to an exposed Bulkamid agent at its injection site.
Exposed intravesical Bulkamid can act as a foreign body with lithogenic potential to cause urinary bladder stone formation. This article highlights urinary bladder stone formation as a late potential complication of Bulkamid periurethral injection.
Key Points
1. Urethral bulking therapy has provided a minimally invasive alternative to treating mixed/stressed urinary incontinence with limited short- and medium-term complications. There is, however, little publication on long-term complications associated with bulking therapy.
2. The authors describe an unexpected finding of a bladder stone adhered to an exposed urethral bulking agent, Bulkamid® (Axonics, Irvine, California, USA), which was injected 10 years prior. Bulkamid is non-toxic and inert in tissue; however, once exposed to intravesical urine for prolonged periods, it acts as a foreign body and a nidus for calculi formation.
3. Exposed bulking agents, extruding from its injection sites, have the potential for urinary stone formation with prolonged urine exposure. Care should be taken to ensure adequate depth of Bulkamid injection, to prevent later extrusion and complications.
INTRODUCTION
Polyacrylamide hydrogel (PAHG) Bulkamid® (Axonics, Irvine, California, USA) was introduced in Europe as a promising periurethral bulking agent for the treatment of female stress and mixed urinary incontinence in 2006.1 Composed of 2.5% polyacrylamide and 97.5% water, it benefits from being non-biodegradable and stable long-term. Its efficacy is widely published, with growing interest as a minimally invasive treatment option. Subjective symptomatic improvement rates have been reported to be as high as 82.8% at 3-year (3–60 months) follow-up.2 Its side effect profile includes mild to self-limiting symptoms. However, there are limited data available on the long-term safety of this bulking agent injection. Here, the authors highlight an unexpected 10-year late complication of Bulkamid periurethral bulking injection.
CASE REPORT
An 80-year-old female patient was referred to the urology clinic with recurrent cystitis in October 2020. They previously had Bulkamid periurethral injection under local anaesthesia in 2009 with good outcomes; however, over the recent 9 months, the patient presented with dysuria, increased urinary frequency and urgency, and subsequently became incontinent, especially during the last 3 months. Urine tests and cultures confirmed Escherichia coli infection, which was resistant to trimethoprim and amoxicillin. Despite having repeated courses of antibiotics, the patient remained symptomatic. The patient was otherwise in excellent health, with no significant comorbidities.
INVESTIGATIONS
Urinary bladder ultrasound imaging revealed a single 2 cm mass with acoustic shadow, suggestive of a bladder stone. There was no significant postvoid residual volume. Subsequent flexible cystoscopy confirmed the presence of a stone, which was fixed to the previous Bulkamid injection site at 9 o’clock of the bladder neck. The bladder mucosa close to the trigone was inflamed, but the rest of the bladder looked healthy, with no signs of chronic cystitis. The patient was then given further antibiotic treatment and put on the waiting list for litholapaxy.
TREATMENT
As planned, the patient was admitted a few weeks later and had the urinary bladder stone removed under general anaesthesia. From the findings, the 2 cm stone (Figure 1) was adherent to the exposed Bulkamid agent at the bladder neck. This was dislodged and fragmented with lithoclast; all stone fragments were removed. The remnant exposed Bulkamid at the 9 o’clock site was also completely removed endoscopically (Figure 2). The patient’s urinary symptoms and urinary tract infection resolved post-operatively with the completion of an antibiotic course.
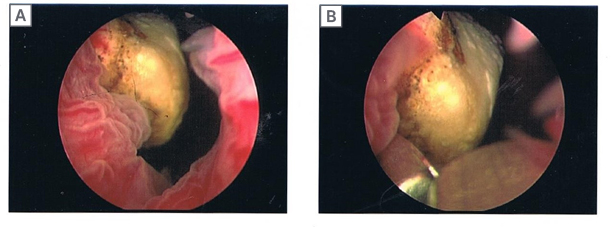
Figure 1: Endoscopic images of a urinary bladder stone at the bladder neck.
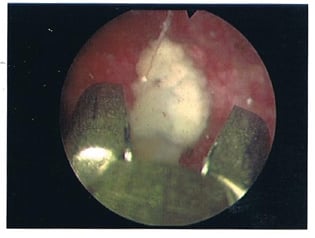
Figure 2: Endoscopic image of exposed Bulkamid® (Axonics, Irvine, California, USA) bulking agent at the urinary bladder neck.
DISCUSSION
Periurethral bulking injections are a less invasive alternative treatment for female stress urinary incontinence compared to the gold standard treatment of surgical repair with a synthetic mid-urethral sling (MUS), with its long-term efficacy and durability. In a recent systemic review comparing both treatment options, MUS was two- to five-times more likely to result in a cure compared with periurethral bulking injections.3
There has, however, been a growth in controversies surrounding the long-term complications of MUS, including long-term pain, urethral erosion, and medical-legal implications. This has led to an increasing shift towards the use of bulking agents in recent years.4
There are currently a variety of bulking agents marketed in different countries. While no study directly compares polyacrylamide hydrogel (Bulkamid) to the other bulking agents, its safety and effectiveness profile has been demonstrated in numerous short- and medium-term studies.5
Polyacrylamide hydrogel has widely been used in other medical specialities, including breast augmentation and facial fillers for cosmesis. Its suitability for in vivo soft tissue injection is owed to its non-biodegradability, non-toxicity, and resistance to migration and calcification long-term.6
Despite its safety profile, Bulkamid injection for stress urinary incontinence has some reported adverse effects. The most frequently reported adverse effects were pain at the injection site and urinary tract infections. This was followed by haematuria and transient urinary retention. One study reported a serious adverse effect of a periurethral abscess.7
To the authors’ best knowledge, there were no reported cases of urinary bladder stones associated with Bulkamid periurethral injections until this case. While the reason for this stone formation is unclear, this article postulates a number of possible mechanisms for stone formation based on the exposed bulking agent.
Virtually all foreign bodies in the urinary bladder are lithogenic with the potential to act as a nidus for stone formation. This is seen regularly with retained iatrogenic and non-iatrogenic objects, including ureteric stents, long-term urinary catheters, retained suturing materials, and inserted domestic items.8 While Bulkamid has demonstrated long-term resistance to calcification within soft tissue in a previous study,6 there are no experimental reports with long-term exposure of Bulkamid to urine. Bulkamid exposure to the intravesical urine long-term may have the potential to accumulate minerals from urine over time, thus allowing for layered stone formation. This is evident in this case with the stone’s adhesion to the exposed Bulkamid agent.
Secondly, this patient presented with recurrent cystitis and confirmed antibiotic-resistant E. coli, a bacteria capable of forming biofilm. Bacteria and biofilm on a foreign body in the urinary system create an environment preferential for calcium and struvite stone formation through ureolysis.9 Unfortunately, stone analysis was not performed in this case to confirm the composition.
To avoid the complication of bulking agent extrusion from the injected urethral site, proper technique must be adhered to. Bulking agents can be injected either via a periurethral or transurethral route, both of which have similar efficacy and safety outcomes.10 Emphasis is placed on slow advancement of the needle to avoid accidental urethral mucosa injury, while ensuring adequate depth in the submucosa. The recommended number of submucosa injection sites is three or more, usually at 3, 6, and 9 o’clock, and within 1 cm distal to the bladder neck. Good coaptation of the urethral wall is achieved with no more than 0.5 ml of Bulkamid injected at each of the injection sites.2
CONCLUSION
The authors report an unexpected finding of a urinary bladder stone adhered to an exposed Bulkamid agent as a late complication of periurethral bulking injection. Given the limited data available on the long-term safety of Bulkamid, this is a potential complication to be considered in patients presenting with new and recurring symptoms years after undergoing periurethral injection. The injection procedure should aim for adequate submucosal depth to reduce the risk of Bulkamid extrusion, and thus prevent subsequent stone formation.








