Abstract
Urethral reconstruction for complex conditions remains a challenge because of the unsatisfactory long-term results and problems associated with the harvesting of adequate replacement tissues. Tissue engineered substitutes, either scaffolds alone or in combination with cells, can overcome some of the aforementioned problems. Currently, such tissue engineered substitutes have been gaining popularity, as evidenced by >80 published preclinical and 20 clinical studies. This review summarises the currently available literature on the cell-based tissue engineered substitutes (11 studies) for urethral reconstruction. Clinical translational challenges and future directions are also discussed.
INTRODUCTION
Normal urethral function can be severely impaired by congenital or a variety of acquired urethral conditions, such as hypospadias, strictures, fistulas, trauma, and cancer. Numerous reconstructive techniques have been developed to restore urethral function in such conditions. Despite this progress, reconstruction for addressing strictures and hypospadias still poses problems for urologists. Availability of adequate replacement for long urethral defects or the poor quality of the urethral plate remains a challenge. Reconstruction in such cases requires the use of additional tissue, such as local genital tissue flaps from preputial,1 penile,2 and scrotal skin,3 or testicular tunica vaginalis,4 or extragenital tissue grafts from skin,5 buccal mucosa,2 lingual tissue,6 or colonic mucosa.7 However, these techniques are not effective for long or complex strictures, and long-term follow-ups have demonstrated significant complications and decreased quality of life.2 The major complications at the operation site include recurrence of stricture and fistula formation.2,4,6,7 Complications also occur at the donor sites, such as difficulty in opening the mouth,8 numbness,8,9 discomfort,9 nerve damage,10 bleeding, and haematoma.11 Also, donor tissue is limited for cases of panurethral strictures or recurring strictures, where tissues were harvested previously. Tissue engineering (TE) may overcome some of the aforementioned problems. Efficacious in vitro-engineered urethral tissue and off-the-shelf substitutes will represent a significant step in advancing urethral reconstructive surgery. Currently, there are >80 published preclinical and 20 clinical studies for urethral TE substitutes.12 In this review, we have evaluated currently available clinical studies regarding the potential of TE in urethral reconstruction; in particular, those describing the use of cells alone and in combination with scaffolds in humans. Translational challenges with such products and the possible future developments in this field are also discussed.
CELL-BASED TISSUE ENGINEERING OF THE URETHRA
TE aims to replace damaged tissues and organs and restore function by combining the fields of cell biology, materials science, and engineering.1-4 Two strategies are employed in urethral TE: the first strategy is scaffold-based, in which natural or synthetic materials are used as grafts and urethral regeneration depends on the body’s ability to heal; the second strategy is cell-based, in which urethral substitutes are engineered in vitro by cells alone or by combining cells with natural and/or synthetic scaffolds. An overview of urethral TE options is described extensively in the literature.12-16
Reconstructing urethras with various acellular natural and synthetic materials has been found to be safe and effective in animals.12 Clinically, naturally derived off-the-shelf materials, such as small intestinal submucosa, bladder acellular mucosa, acellular dermis, and urethral acellular matrix were found to be safe, and demonstrated success rates of >75% in the majority of cases.12,17 Failures were common in patients with longer strictures (>4 cm),18,19 in those with penile or penile-bulbar strictures,20 and in those with previous urethroplasty (unhealthy urethral bed, unsatisfactory vascularity).21
Cell-based TE substitutes overcome the limitations of acellular grafts by lengthening the distance over which regeneration occurs. Various cell and scaffold combinations were evaluated in animals with good success.12,16 A meta-analysis of cell-based TE substitutes for urethral reconstruction in animals revealed that these grafts were 5.7-times better than unseeded grafts.16 In another meta-analysis, cells significantly reduced the probability of strictures, stenosis, fistulas, and infections independent of the scaffold type.12
Despite multiple animal studies, only a few clinical studies of cell-based TE substitutes for urethral reconstruction in hypospadias and stricture have been published (11 studies, Table 1). PubMed and Google Scholar were queried for synonyms of TE (e.g., regenerative medicine and cells) and synonyms of urethral reconstruction (e.g., urethral repair and urethroplasty) to identify these clinical studies.
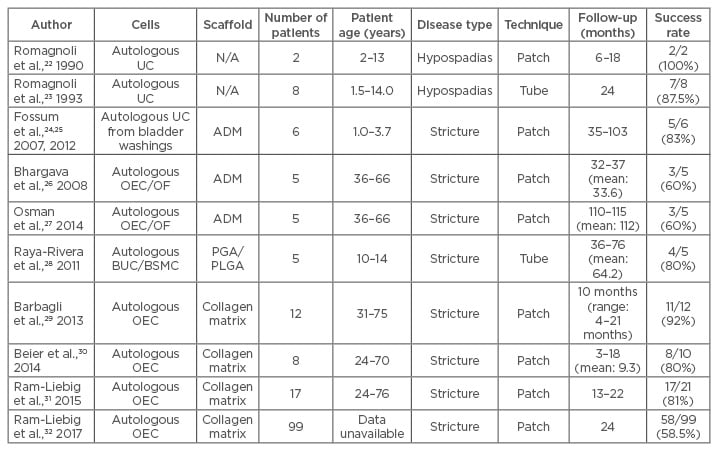
Table 1: Cell-based tissue engineered substitutes used in urethral reconstruction.
ADM: acellular dermal matrix; BSMC: bladder smooth muscle cell; BUC: bladder urothelial cells; N/A: not applicable; OEC: oral epithelial cells; OF: oral fibroblast; PGA: polyglycolic acid; PLGA: poly(lactic-co-glycolic acid); UC: urothelial cells.
Romagnoli et al.22 constructed neourethras in two posterior hypospadias patients with autologous stratified urethral epithelial sheets. In one patient this cell sheet was grafted onto the tissue bed, which had been exposed by incising the penile ventral surface. The neourethra, which exhibited organised stratified epithelium 10 days post-surgery, was tubularised and linked to the meatus after 6 months. The patient voided normally; however, fistulas developed on the ventral surface of the neourethra and at the junction of the neourethra and the meatus, which were surgically managed. At 1 year, normal urethral mucosa with stratified squamous epithelium was observed. In the second patient, the new urethra was tubularised and immediately connected to the meatus after 10 days. Fistulas developed at the junction of the new urethra and the original meatus. Stenosis at the coronal sulcus of the glans was also observed. Fistulas and stenosis were managed by surgery and dilation, respectively. Follow-up after 6 months demonstrated normal urinary and erectile functions and no stricture or fistulas.
Romagnoli et al.23 modified their two-stage procedure into a one-stage procedure by directly implanting tubular autologous epithelial sheets in eight severe proximal hypospadias patients. The tubularised grafts were inserted into the subcutaneous tunnel prepared by dissection of the urethral meatus up to the fossa navicularis of the glans. The patients were followed for up to 2 years. Endoscopy was performed in all patients and biopsies were taken from four patients. One patient developed a fistula within 20 days. All patients exhibited stenosis at the coronal sulcus of the glans, which was managed by dilation. The mucosa was smooth with stratified epithelium.
Fossum et al.24 used autologous urothelial cell-seeded acellular dermis in six males with severe scrotal or perineal hypospadias and pronounced chordee. Urothelial cells were harvested from bladder washes during Stage 1 of penile straightening. In Stage 2, TE graft was implanted in an onlay fashion with the cells facing the lumen. Cosmetic appearance, voiding function, urinary flow, urethroscopy, and histopathology were assessed for 3.0–5.5 years. Cosmetic appearance was considered good in all cases. The meatus had an adequate opening at or near the glans in all cases. All six males voided through their neourethras without straining and had no residual urine. Five patients voided in a standing position and presented bell-shaped flow curves. Restricture occurred at 12 months in one patient, which was managed by urethrotomy. Another developed a proximal anastomosis obstruction, which was managed with stenting. Two males developed fistulas, which were surgically corrected. All patients had a wide neourethra with smooth mucosa. Mucosal lining with urothelial cells were seen in three cases. Further long-term follow-up (6–8 years) showed that all six patients still had good cosmetic appearance, good voiding function, and straight artificial erections, with ingrowth of urothelium or squamous epithelium on the transplanted site.25
Bhargava et al.26 reported the 3-year clinical results of TE urethroplasty for complex lichen sclerosis strictures. The TE graft with stratified epithelium was constructed with autologous oral epithelial cells and fibroblasts seeded onto acellular dermis. Both two-stage (n=3) and one-stage (n=1) procedures were performed. Visual examination and endoscopy was performed at follow-up. Of the two-stage patients, one patient underwent a complete graft excision due to fibrosis with chordee at 8 months, the second underwent a proximal graft excision due to hyperproliferation at 9 months, and the third had sub-meatal stenosis that required dilation. The excised parts were substituted with oral mucosa. Both the one-stage patients developed stricture at the bulbar anastomotic site within 9 months; these were managed with urethrotomy. Osman et al.27 reported the 9-year clinical results in these patients. Three patients continue to use intermittent self-dilation. All patients had a patent and normal urethra.
Raya-Rivera et al.28 reported the effectiveness of tubularised TE urethras in five boys. Three of the boys presented with a complete posterior stricture, and two had previous failed posterior urethroplasty. Autologous muscle cells and urothelial cells were isolated from the bladder biopsy and expanded. The muscle cells were seeded on the outer surface and epithelial cells on the inner surface of tubularised polyglycolic meshes coated with poly(lactic-co-glycolic acid). These tubes were surgically implanted after stricture excision. The boys were followed for up to 6 years. Voiding function, urinary flow, urethroscopy, urethrogram, urethral biopsies, and questionnaire outcomes were assessed. All patients voided normally without dysuria, straining, or dribbling. None developed strictures or fistulas. All patients maintained wide urethral calibres. Neourethral histologic characteristics were similar to native urethra at 3 months and had no aberrant changes over time. One patient exhibited narrowing at the proximal graft anastomotic site that required incision.
MukoCell® (Urotiss Europe GmbH, Dortmund, Germany), an autologous cell transplant that uses oral epithelial cells in a collagen matrix, is a commercial TE graft available to German patients. The exact cell culture protocol and composition of the scaffold is unknown. One study assessed twelve patients with idiopathic uncomplicated bulbar urethral stricture who were treated with MukoCell. At a mean follow-up of 10 months, stricture reoccurred in one patient.29 Ten patients with bulbar strictures were treated with Mukocell. At a mean follow-up of 9.3 months, strictures reoccurred in two patients.30 Twenty-one patients with bulbar stricture (n=18) and proximal penile and bulbar stricture (n=3) were treated with MukoCell by a ventral onlay procedure.31 Seventeen of these patients had some previous instrumentation. The median follow-up was 18 months. Seventeen patients were stricture free and urethrography showed a wide and patent urethra. The mean maximum peak flow increased from preoperative 9.9 mL/s to postoperative 30.0 mL/s. Restricture occurred by 11 months following urethroplasty. In one of these patients, the restricture occurred outside of the transplanted graft area. The failed procedures were treated successfully using either urethrotomy, dilatation, or meatoplasty.
Ram-Liebig et al.32 recently published the 2-year safety and efficacy results of MukoCell for urethral strictures of various aetiology, location, and severity in a multicentre prospective observational trial. The urethroplasty was performed in eight German centres with various levels of experience. A total of 99 patients were included in this study. Except for one, all of the patients had multiple previously failed surgical interventions. Statistical analysis was also performed to identify risk factors for stricture recurrence. The success rate, defined as the absence of stricture recurrence, was 67.3% at 12 months and 58.2% at 24 months. The success rate was dependent upon surgeon skill and experience. The success rate varied between 85.7% in the case of high experience and 0.0% in the case of low experience. Timing of catheter removal and number of prior surgeries were identified as independent risk factors for stricture recurrence. Patients with catheter removal ≥28 days postsurgery had the highest risk of stricture recurrence, as did patients with four or more prior treatments.
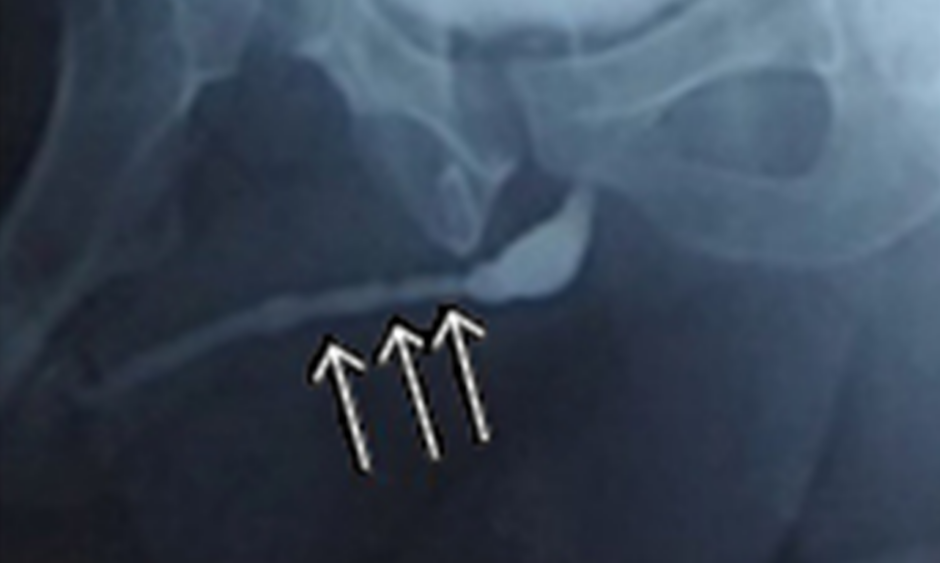
The authors have also used TE to treat a single case of lichen sclerosis stricture in a 45-year-old with previous failed urethroplasty. Rangadore Memorial Hospital Ethics Committee, Bangalore, India, approved this study. A urethrogram showing stricturing in the anterior urethra can be seen in Figure 1A. Autologous oral epithelial cells were seeded onto denuded amniotic membrane mounted on a polypropylene mesh. This TE graft was monitored daily to examine cell morphology, proliferation, and sterility. Once the cells were confluent and found to be free of microbial and mycoplasma contamination, it was ready for transplantation. The fibrosed urethra was exposed ventrally until the healthy urethral tissue was reached proximally. After haemostasis, the TE graft was applied with cells facing the raw area and fixed at edges to the exposed corpus spongiosum (Figure 2A and 2B). A catheter was inserted for voiding of urine via a proximal urethrostomy. The graft area was covered with a sterile dressing, which was removed after 2 days; the catheter and mesh were removed 4 days later. The grafted area was observed periodically for a period of 6.5 months to assess healing and epithelialisation. The graft area healed well, with complete re-epithelialisation (Figure 2C and 2D), and was elastic. No adverse reaction to the graft was observed. A biopsy taken from the healed area confirmed the presence of stratified epithelium. The urethra was then tubularised and the penis reconstructed. The patient was able to void normally without any pain. After 1 year, cystoscopy revealed smooth and continuous urethral mucosa (Figure 1C) with narrowing at the anastomotic site. A urethrogram at 2 years showed that the repaired urethra was still patent but with narrowing at the anastomotic site (Figure 1B).
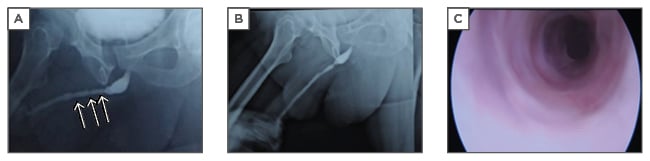
Figure 1: A) Urethrogram showing the narrowing (arrows) in the anterior urethra; B) urethrogram after 2 years showing the narrowing at anastomotic area; C) cystoscopy of the reconstructed urethra showing smooth and patent lumen with narrowing at the anastomotic site.
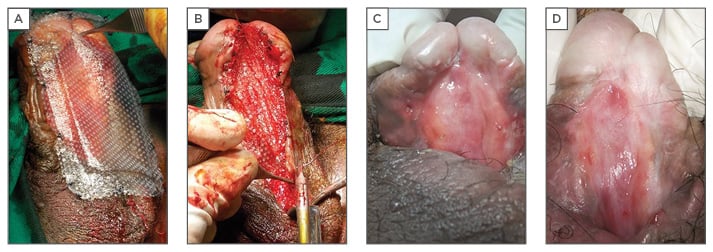
Figure 2: A) Placement of tissue-engineered graft on the excised area of the urethra; B) tissue-engineered graft affixed to the edges with sutures; C) healing of urethra 87 days after tissue-engineered graft; D) healing of urethra 193 days after tissue-engineered graft.
All of the above studies demonstrate the feasibility and efficacy of using TE-based approaches for the treatment of urethral disease. Nonetheless, significant challenges remain.
TRANSLATIONAL CHALLENGES
Only a few TE products have been used clinically despite a plethora of preclinical studies suggesting that multiple challenges hamper the transition of TE products from bench-to-bedside. Some of these challenges include appropriate preclinical or clinical studies, regulations, cost, patient population, manufacturing facility, variability, off-the-shelf availability, surgical techniques, among others.
One major translational challenge is that the level of evidence from preclinical studies is low-to-moderate. The efficacy of TE products is evaluated in healthy animals that do not replicate the pathophysiology of urethral strictures and hypospadias. The graft uptake and the healing process in the normal urethral bed would be completely different from the diseased urethra. In such healthy animals, TE products might be more efficacious because of the presence of a vascularised healthy urethral bed. Furthermore, in many animal studies, unseeded scaffolds were considered as controls, not sham or standard treatment.12,16 Susceptibility to publication bias may have also lead to overestimation of the treatment effect in preclinical studies. Most of the clinical studies have treated only a few patients, and hence a high level of evidence is not possible.
To ensure patient safety, quality, and efficacy, there are multiple regulatory oversights for TE therapies at all stages, starting from in vitro studies all the way to commercialisation. Unfortunately, regulatory pathways vary in different countries. The regulatory pathways are sometimes not able to keep up with the rapid progress made in TE, and approval processes are time-consuming and expensive. In other instances, TE are regulated by procedures established for conventional pharmaceutical products and are often inappropriate.
The cost of TE when compared to autologous grafts is very high. Currently, oral mucosa successfully repairs simple urethral strictures and hypospadias, so justifying the high cost in such cases is difficult. Hence, TE grafts may be required only in cases of extensive strictures or hypospadias, or in recurrent cases with previously failed graft urethroplasties. However, the complication and failure rates of the TE grafts in such cases could be expected to be high too, putting TE in a difficult position to prove itself. Additionally, the efficacy of the TE grafts can only be established in prospective, multicentred, randomised studies with appropriate control groups. This would be difficult, as the TE are developed for urethral conditions where current treatment is not satisfactory. Also, conducting such studies is expensive and time-consuming.
Unlike drugs and biologics, which have distinct reproducible properties, cells vary depending on the patient, further hampering translation of cell-based therapies. In addition, the patient’s own genetic makeup can also influence many aspects, including, for example, the healing milieu. This issue may be even more relevant in patients with urethral strictures secondary to lichen sclerosis because it may be a genetic issue.
Another limiting factor is the long time required for the preparation of cell-based TE grafts and, at present, it is not suitable as an off-the-shelf product.
Furthermore, an important challenge is the TE graft placement technique used. Clinically, reconstructive surgeons have a variety of techniques that may be used according to factors such as aetiology, stricture position, and length. TE grafts should be demonstrated to be efficacious in all surgical techniques.
FUTURE DIRECTIONS
Robust assessment and evidence of successful TE grafts in animal models is crucial and mandatory before clinical testing. Currently, a few animal models are available for urethral diseases, but they do not fully recreate the complexity and compromised vascularity.33-35 Hence, the development of validated animal models that reproduce these aspects faithfully is critical, and studies in these models with proper experimental design parameters are necessary. Furthermore, such animal models are ideal opportunities for research concerning the pathogenesis and molecular biology of urethral diseases.
Additionally, more basic studies are required to elucidate the molecular mechanisms of urethral stricture, which can lead to the development of better TE techniques that can counteract the stricture. For example, in a fibrosed urethra the expression of connective tissue growth factor is high.36 Hence, TE approaches that can downregulate the expression of connective tissue growth factor can potentially counteract stricture formation. Animal studies allow the assessment of grafts at serial time points, which is not possible in the clinical setting. Hence, development of non-invasive novel imaging modalities that would permit in vivo assessment of TE grafts would be helpful. Regeneration of corpus spongiosum (CS), which is fibrosed in urethral stricture disease37 and partially absent in hypospadias,38 needs further investigation. Current TE grafts are geared towards regenerating the epithelium and not the CS. The CS provides mechanical support and blood supply to the urethra39 and thus it is hypothesised that, due to the absence of CS, urethral reconstructions may fail in the long term.40 Therefore, engineered spongiosum tissue together with urethral mucosa may provide a better blood supply and mechanical protection of the urethra and better functional outcome. Another technique that should be investigated is the use of three-dimensional (3D) printing. 3D printing can process multiple cell types and multiple biomaterials simultaneously and so could fabricate complex tissues. 3D printing overcomes the limitations with traditional TE methods; namely, poor control of scaffold microarchitecture, difficulties in homogenous seeding of the scaffold, and inability to spatially distribute multiple cell types.41 3D printing also has the ability to create customised TE grafts for each patient. Investigation into 3D printing for this purpose is underway.42
Further research should be aimed at making these TE strategies truly off-the-shelf. This can be achieved with the use of allogeneic cells that can be either seeded on scaffolds and cryopreserved or delivered to the defect site at the time of surgery by mixing them with novel biomaterials to form in situ paste or hydrogels. Further research should also be aimed at developing minimally invasive techniques for TE urethroplasty. One such technique could be excision of strictures endoscopically with the use of a laser and applying the off-the-shelf allogeneic cell paste to the excised area for tissue regeneration.
Continuous collaboration among various disciplines, such as developmental biology, cell biology, biomaterial science, and surgery, are needed to further advance urethral TE.
CONCLUSIONS
Cell-based TE urethral substitutes have encouraging clinical results; however, more clinical experience is warranted.