Abstract
Radical cystectomy with extended pelvic lymph node dissection is the gold standard for the treatment of muscle-invasive bladder cancer (MIBC). Despite this definitive surgery, patients face a recurrence rate of approximately 50% 5 years after surgery. This high recurrence rate may be related to micrometastatic disease at the time of the surgery. Although the data to support adjuvant chemotherapy for treatment of these patients are insufficient, neoadjuvant chemotherapy (NAC) that includes cisplatin-based combination therapy for MIBC is recommended by the guidelines. This article reviews the current situation in NAC for the treatment of MIBC.
INTRODUCTION
Bladder cancer is one of the most common types of urinary tract malignancy representing the fourth most common cancer in men, with men accounting for 80% of all bladder cancer patients, and the eighth most common cancer in women.1 Bladder cancer is a worldwide problem, with an estimated 429,800 new cases of bladder cancer and 165,100 deaths caused by the disease occurring in 2012. Incidence rates are highest in Europe, Northern America, Western Asia, and Northern Africa.2 Approximately 90% of bladder cancers are the transitional cell or urothelial carcinoma type, and at initial diagnosis, 30% are found to be muscle-invasive bladder cancer (MIBC). Radical cystectomy and pelvic lymph node dissection remains the gold standard treatment for patients with MIBC.3 The risk of recurrence following radical cystectomy for the treatment of MIBC is high, and correlates with stage.4 Although radical cystectomy is the gold standard, it only provides 5-year survival in approximately 50% of patients.5,6 Despite this being the gold standard treatment, patients with MIBC have about a 50% rate of recurrence.6
The risk of recurrence after radical cystectomy for clinically localised bladder cancer is high and stage dependent. Some authors suggest that the predominant cause of this high recurrence rate is occult micrometastases present at the time of radical cystectomy.1 As a result, neoadjuvant chemotherapy (NAC) has been used to remove these occult micrometastases and improve unsatisfactory results for the last three decades.
In this review, we will discuss the current state of NAC for the treatment of MIBC. Only the results of randomised controlled clinical studies and meta-analyses for NAC have been reported in this article.
ADVANTAGES AND DISADVANTAGES OF NEOADJUVANT CHEMOTHERAPY
NAC has been administered to patients with MIBC as part of bladder-preserving strategies aiming to both eliminate systemic microscopic disease early, and improve cancer-specific survival.7 There are many advantages of administering NAC before radical cystectomy to patients with MIBC and cN0 M0. However, there are also some disadvantages of NAC, which cause most urologists to hesitate about its use. Although the data to support adjuvant chemotherapy are insufficient, NAC, which includes cisplatin-based combination therapy, is recommended for the treatment of MIBC by the guidelines on muscle invasive and metastatic bladder cancer from the European Association of Urology.5 Although only 1.2% of patients with MIBC cancer received NAC according to the data of the American National Cancer Data Base between 1998 and 2003,8 this rate was reported as 12% by Feifer et al.9 in 2011. Despite the recommendations associated with the use of NAC in patients with MIBC, and the outcomes of various randomised trials, the rate of patients receiving NAC has seen little increase.5
The potential advantages of NAC include:5
- Chemotherapy is delivered at the earliest time point for the treatment of micrometastatic disease
- It provides the opportunity to assess the chemosensitivity in vivo
- Patients have a higher tolerance and compliance to chemotherapy before radical cystectomy
- Patients may respond to NAC and reveal a suitable pathological status, determined mainly by achieving pT0, pN0, and negative surgical margins
The disadvantages of NAC include:5
- The definitive therapy delays in patients not sensitive to chemotherapy
- NAC may negatively affect surgical mortality and morbidity
- Overtreatment is a possible negative consequence for some patients
There is in fact no evidence for the first two disadvantages of NAC in the literature. Although published trials regarding the negative impact of delayed radical cystectomy only include series of chemo-naïve patients, delayed curative surgery might comprise the surgical results in patients not sensitive to chemotherapy. There are no studies showing that delayed radical cystectomy due to NAC has an adverse effect on survival in the literature.5 In 2015, a retrospective study regarding delayed cystectomy was published. The impact of the timing of radical cystectomy from diagnosis of MIBC on survival in patients treated with NAC and radical cystectomy was evaluated in this study. The study showed that the timing of radical cystectomy in relation to the date of MIBC diagnosis did not significantly impact overall survival in patients with MIBC receiving NAC.10
Only one study, published in 2003, has reported a negative effect on surgical mortality and morbidity associated with NAC.1 In this study, the survival and surgical outcomes of patients (N=317) treated with radical cystectomy and NAC (combination group) were compared with radical cystectomy alone (cystectomy group). They found that planned radical cystectomy was performed in 82% and 81% of the patients in the combination and cystectomy groups, respectively. There were no significant differences between the two groups in the rate of Grade 2 or 3 post-surgical complications, or deaths. Although there were no significant differences between the two groups in the rates of surgical mortality and morbidity, the median survival of the combination group (77 months) was longer than the cystectomy group (46 months). The percentage of surviving patients at Year 5 was 57% and 43% in the combination and cystectomy groups, respectively. Similarly, the data of the combined Nordic trial (N=620) showed that NAC did not have a significant effect on the rate of performable radical cystectomy. The cystectomy frequencies of the experimental and control groups were 86% and 87%, respectively.3
Overtreatment is a major problem associated with NAC as clinical staging of MIBC using bimanual examination, magnetic resonance imaging, or computed tomography may often result in over or understaging, and have a staging accuracy of only 70%.11 Characterising responders to NAC is very important to minimise overtreatment and the unnecessary delay of curative therapy in MIBC. For patients with a complete response to NAC (pT0 N0), treatment has a major positive affect on overall survival.12 Identification of responders to NAC utilising tumour molecular profiling in specimens obtained via transurethral resection of the bladder may guide the use of NAC.13 Many studies have investigated imaging techniques using the early identification of responders. The results of these studies showed that magnetic resonance imaging, computed tomography, or positron emission tomography could not accurately predict response to NAC.14-16
NEOADJUVANT CHEMOTHERAPY REGIMENS
Many chemotherapy regimens have been used for the treatment of MIBC before radical cystectomy. The guidelines recommend the use of platinum-based combination chemotherapy regimens as neoadjuvant therapy in those patients.5 The combination regimens tested were methotrexate, vinblastine, adriamycin (doxorubicin)/(epirubicin), and cisplatin (MVA[E]C); cisplatin, methotrexate, and vinblastine (CMV); cisplatin and methotrexate (CM); cisplatin/adriamycin, cisplatin, and 5-fluorouracil (5-FU); and carboplatin, methotrexate, and vinblastin. MVAC is the only regimen with Level I data at this time.1 The most current retrospective and pooled studies have reported that efficacy is similar between gemcitabine, cispaltin (GC), and MVAC.17-20 The data from these studies showed that although there were no differences between the response rate to GC and MVAC, the rate of chemotherapy-related complications, such as anaemia, neutropenia, neutropenic fever, and mucositis in patients receiving GC were significantly decreased. However, there have been no randomised prospective studies comparing GC with MVAC regimens in the literature.
RANDOMISED CLINICAL TRIALS AND META-ANALYSES FOR NEOADJUVANT CHEMOTHERAPY
Several randomised Phase III trials have published outcomes of NAC for the treatment of MIBC.1,21-28 The data of these studies are summarised in Table 1. Shipley25 and Abol-Enein26 evaluated the impact of two or three cycles CMV on overall survival at 5 years. However, these studies found that there were no statistically significant benefits of chemotherapy. Sengelov24 and NORDIC II23 investigated the effectiveness of three cycles CM before surgery or radiotherapy. The results of these two studies were similar to the results of earlier studies; there were no differences in overall survival between the experimental and control arms. Wallace27 and Martínez-Piñeiro28 used three cycles of cisplatin alone as NAC in their studies, and reported no significant benefit of this course of treatment. Despite the negative results reported by earlier randomised controlled studies, the results of two studies1,21 and three meta-analyses29-31 have successfully demonstrated a survival benefit in the use of NAC for the treatment of patients with MIBC. The most recent and largest study was published by the International Collaboration of Trialists, and included 976 patients with MIBC. This study demonstrated a statistically significant 16% reduction in the risk of death (hazard ratio [HR]: 0.84; 95% confidence interval [CI]: 0.72–0.99; p=0.037), corresponding to an increase in 10-year survival from 30% to 36% after NAC. Many randomised controlled studies have shown a statistically insignificant increase in overall or disease-free survival at 5 years. Grossman et al.1 randomised patients with MIBC to either NAC plus cystectomy or cystectomy alone. Patients in the experimental arm received three cycles MVAC before cystectomy. Median survival in the experimental and control arms were 77 and 46 months (p=0.05), respectively, and overall survival was 57% and 43% at 5 years (p=0.06), respectively.
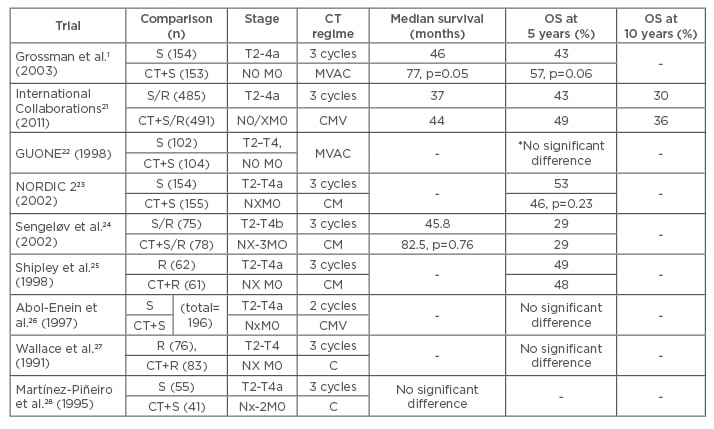
Table 1: Randomised Phase III trials with regard to neoadjuvant chemotherapy for the treatment of muscle-invasive bladder cancer.
*overall survival at 3 years
CT: chemotherapy; S: surgery; R: radiotherapy; MVAC: methotrexate, vinblastine, doxorubicin/epirubicin, cisplatin; CMV: cisplatin, methotrexate, vinblastine; CM: cisplatin, methotrexate; C: cisplatin; OS: overall survival.
The three most recent meta-analyses with regard to NAC for the treatment of MIBC were published between 2003 and 2005. The Advanced Bladder Cancer Meta-analysis Collaboration29 analysed updated data for 2,688 individual patients from 10 randomised controlled trials. The results of the first meta-analysis showed that platinum-based combination chemotherapy provides a significant benefit to overall survival (HR: 0.87, 95% CI: 0.78–0.98, p=0.016). It was also demonstrated that NAC provides a 13% reduction in risk of death, 5% absolute benefit at 5 years, and an increase in overall survival from 45% to 50%.28 The second meta-analysis was comprised of the data of 2,605 patients with MIBC from 11 randomised controlled trials. They noted an absolute overall survival benefit of 6.5% (95% CI: 2–11%) from 50% to 56.5% regarding platinum-based combination chemotherapy.30 The last meta-analysis for NAC analysed the data of 3,005 patients in 11 randomised controlled trials.31 They noticed significant overall and disease-free survival benefits with regard to platinum-based combination chemotherapy (HR: 0.86, 95% CI: 0.77–0.95, p=0.003 and HR: 0.78, 95% CI: 0.71–0.86, p<0.0001, respectively). These are equivalent to a 5% and 9% absolute improvement in overall survival and disease-free survival at 5 years, respectively. They consequently reported that their findings provided the best available evidence in support of the use of NAC for the patients with MIBC cancer.
More modern chemotherapy regimens, such as GC, have shown similar pT0/pT1 rates as MVAC in recent retrospective series and pooled data analyses, but have not been used in randomised controlled trials. Dash et al.19 retrospectively investigated patients with MIBC who received neoadjuvant GC before radical cystectomy. They suggested that neoadjuvant GC is feasible and allows for timely drug delivery. They also reported that the proportion of GC-treated patients, whose primary tumours were downstaged, with prolonged disease-free survival and minimal or no residual disease, was similar to MVAC-treated patients. Lee et al.18 compared pathologic outcomes after treatment with GC versus MVAC in the neoadjuvant setting. They observed similar pathologic response rates for GC and MVAC in this cohort of patients with MIBC. This study supports the use of GC as an alternative regimen in the neoadjuvant setting. Yuh et al.17 evaluated the effectiveness of neoadjuvant GC for MIBC based on currently published studies. They reported that GC yielded appreciable pathological response rates in patients with MIBC. They also suggested that since pathological response has been implicated as a potential surrogate for survival in MIBC, these data suggested that neoadjuvant GC might warrant further prospective assessment.
Adjuvant chemotherapy for the treatment of patients with MIBC (pT3/pT4 and/or N+ M0) is under debate and still infrequently used. There is limited evidence from adequately conducted randomised Phase III trials in favour of the routine use of adjuvant chemotherapy. These data were not convincing enough to give an unequivocal recommendation for the use of adjuvant chemotherapy.32-34 In contrast, Tjokrowidjaja et al.35 presented the survival benefits of adjuvant and NAC in MIBC in the 2013 ASCO Annual Meeting. They analysed the data of 21 randomised controlled trials (9 adjuvant and 12 NAC) and presented the results of the meta-analysis. They consequently noted that chemotherapy, both adjuvant and neoadjuvant, improves survival in MIBC.
CONCLUSION
Platinum-based NAC for treatment of patients with MIBC is well tolerated (with minimal-to-moderate morbidity and no mortality) and improves overall survival (5–8% at 5 years). Limitations exist in regard to patient selection, current development of surgical techniques, and current chemotherapy combinations, though there is a major impact on overall survival in responders who show complete response (pT0 N0). However, no techniques are available to choose patients who have a higher probability of benefitting from NAC. Consequently, the guidelines and meta-analyses on the treatment of MIBC recommend using NAC (cisplatin-based combination therapy) for patients with T2-T4a, cN0 M0 bladder cancer. Despite the recommendations associated with the use of NAC in patients with MIBC and the outcomes of randomised trials, the rate of patients receiving NAC has seen little increase. The reason for this low rate might be due to some concerns from urologists, such as delayed curative surgery treatment, and negative impact on peri and post- operative mortality and morbidity of chemotherapy. However, there is much evidence in the literature that show that NAC is not associated with these negative outcomes. Therefore, the rate of the use of NAC in patients with MIBC may increase in future.








