Abstract
Peyronie’s disease (PD), which is characterised by fibrous plaque formation in the tunica albuginea of the penis, is associated with pain, erectile dysfunction, and anatomical malformations that negatively affect the quality of life of afflicted men. The optimum medical therapy for PD has not yet been identified. In the last 5 years, commonly used oral medications have been replaced by intralesional therapies. Intralesional collagenase Clostridium histolyticum is the only US Food and Drug Administration (FDA) approved treatment for PD. Minimally invasive intralesional therapies and surgical intervention form the basis of contemporary therapy for this disorder. These therapeutic options, along with selected portions of the guidelines, are explored in this review. The objective is to describe the current state of practice for each of the most commonly used, as well as several developing, treatment modalities of PD.
INTRODUCTION
Peyronie’s disease (PD) is a connective tissue disorder of the penis that is psychologically devastating for affected men and leads to penile deformity. This wound healing disorder is thought to result from trauma or microtrauma to the erect penis in genetically susceptible individuals, though the mechanism of disease has not yet been fully explained.1 Disease prevalence is often quoted at 3.2–8.9% in adult men, though it may be higher due to under-reporting. The most commonly associated comorbidities and risk factors are diabetes, hypertension, lipid abnormalities, ischaemic cardiopathy, erectile dysfunction (ED), low testosterone, smoking, and excessive consumption of alcohol.2-4 Dupuytren’s contracture is more common in patients with PD and affects 9–39% of patients.5-7
The pathophysiological basis for PD relates to the inflammation seen in the acute phase (painful erections, ‘soft’ nodule/plaque) and the subsequent disordered wound-healing, which is characteristic of the chronic phase (disease stabilisation).
The diagnosis of PD involves a focussed history considering the presenting symptoms and erectile function status. Physical examination must include assessment of penile length, palpable nodules, and extent of curvature.8 The assessment of penile curvature occurs during an erection. This can be obtained by a home (self) photograph of a natural erection, which is preferable, or by means of a vacuum-assisted erection test or intracavernosal injection using vasoactive agents.9 Ultrasound measurement of the plaque’s size is inaccurate, and it is not recommended in everyday clinical practice.10 Doppler ultrasound may be required for the assessment of vascular parameters.11
Once the diagnosis is made, the patient should be counselled on the available treatment options. The presence of ED and the related psychological factors may impact treatment strategy.
DISEASE MANAGEMENT OF PEYRONIE’S DISEASE
Non-surgical Treatment
Conservative treatment of PD is primarily focussed on patients in the early stage of the disease (Table 1).
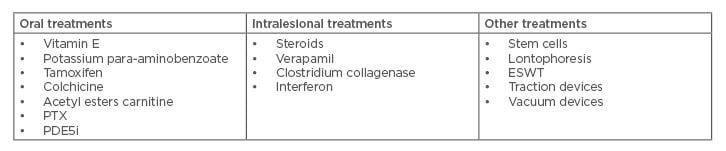
Table 1: Non-surgical treatment options.
ESWT: extracorporeal shock wave treatment, PDE5i: phosphodiesterase Type 5 inhibitor, PTX: pentoxifylline.
Oral Treatment
Vitamin E
Vitamin E has been extensively investigated for its potential use in the treatment of PD. According to guidelines and earlier research, vitamin E is not currently recommended for the treatment of PD.8
Tamoxifen citrate
Tamoxifen citrate is a non-steroidal oestrogen receptor antagonist used in the treatment of breast cancer. This medication has been explored as a therapeutic option for PD due to its inhibitory effects both on the release of transforming growth factor (TGF) from fibroblasts and TGF-receptors.12 As a treatment for PD, tamoxifen is not recommended in the guidelines.8
Potassium para-aminobenzoate
Potassium para-aminobenzoate is an anti-fibrotic agent with monoamine oxidase activity that is used to treat fibrotic conditions, such as Dupuytren’s contracture. Overall, para-aminobenzoate use in PD should be approached with caution given its questionable efficacy and its potentially severe side effects.13
Colchicine
Colchicine is known as a treatment for gout and its proposed mechanism of action in PD is on the basis of its anti-inflammatory effect.14 This medication as a treatment for PD has been abandoned.
Carnitine
Propionyl-L-carnitine is a short-chain acyl derivative of carnitine and acts as an acetyl-coenzyme A inhibitor. Its role in the treatment of PD stems from its anti-oxidant properties, previously utilised for idiopathic infertility, and its antiproliferative effects on endothelial cells.15 Carnitine is currently not recommended in the guidelines for PD.8
Pentoxifylline
Pentoxifylline is a non-specific phosphodiesterase inhibitor with anti-inflammatory and antifibrogenic properties. Pentoxifylline is not recommended in the guidelines for the treatment of PD.8
Non-steroidal anti-inflammatory medications
The clinician may offer oral non-steroidal anti-inflammatory medications to patients suffering from active PD who are in need of pain management.8
Phosphodiesterase Type 5 inhibitors
Phosphodiesterase Type 5 inhibitors (PDE5i) have long been used in the treatment of ED. In PD, these agents are proposed to inhibit tissue remodelling after acute injuries by decreasing oxidative stress responsible for inflammation and fibrosis.16 Therefore, no recommendation can be given for PDE5i in patients with PD.
Topical H-100 gel
H-100 gel is composed of nicardipine, superoxide dismutase, and emu oil. One study demonstrated a significant improvement in flaccid–stretched penile length, curvature, and pain level in 22 patients, thereby opening the door for future research into the suitablility of H-100 gel as a treatment for acute phase PD. H-100 gel is a safe and possibly effective non-invasive, topically applied treatment. A self-limited rash was the only side effect in three patients. However, more safety and efficacy data from larger trials are needed prior to routine usage.17
Stem cell treatment
PD is often associated with antecedent trauma to the erect penis. It is some interplay between trauma and genetic susceptibility that leads to the development of the disease. This idea is supported by the presence of myofibroblasts in PD lesions. These myofibroblasts originate from pluripotent stem cells in the tunica albuginea, but, importantly, they are not present in normal tunica albuginea tissue.18
The therapeutic action of these stem cells is thought to be derived from their proangiogenic capacity that alters the cycle of vascular injury, ischaemia, and fibrosis characteristic of the inflammatory phase of PD.19
Castiglione’s group has been using stem cells to assess improvement of both PD and ED in rat models. Despite the lack of any long-term results, they showed that they could induce PD and ED with TGF-β injections. When they used human adipose tissue-derived stem cells, they noted a reduction in fibrosis and improvement in erectile function.20 Later, Levy’s group published a prospective trial of stem cells in five patients suffering from PD. In this study, 7 of the 10 plaques initially seen with ultrasonography disappeared completely at 3-month follow-up. The results seemed promising.21
While still in its infancy, stem cell therapy offers perhaps the best hope for a definitive PD cure.
Intralesional Therapies
Intralesional injection therapy has been used for years to treat PD. Adequate drug penetration may significantly slow, prevent, or reverse PD plaque formation. Higher concentrations injected immediately into cells should hopefully negate the need for prolonged treatment as seen with some oral medications. Unfortunately, results have been limited and many medications are riddled with local side effects including pain, bruising, and local inflammation.
Intralesional collagenase
Collagenase Clostridium histolyticum (CCh) is an enzyme that degrades interstitial collagen, making it a logical choice in the treatment of PD. It is approved for the treatment of chronic dermal ulcers and severe burns. It has also been successfully used in the treatment of Dupuytren’s contractures, with which PD shares a similar pathophysiology.22
CCh is now approved by the US Food and Drug Administration (FDA) for PD in adult men with a palpable plaque and a curvature deformity of ≥30° at the start of therapy. Intralesional CCh is a purified mix of two collagenases that leads to a breakdown of the collagen when injected into the PD plaque, which can lead to a reduction in penile curvature.23-25
The outcomes of IMPRESS I and II have been published. There were significant results at 1-year follow-up: a mean improvement of 17° in penile curvature at 36 weeks and an improvement in erectile function.26,27
Intralesional treatment with CCh showed significant decreases in the deviation angle, plaque width, and plaque length. CCh is contraindicated for atypical PD patients including ventral plaques and/or ‘hourglass’ deformities.22,28 CCh is associated with minor local side events including penile ecchymosis, swelling, and pain. Serious side effects occurred in approximately 1% of intralesional CCh-treated patients. Corporal rupture (penile fracture) requiring surgical repair was reported as an adverse reaction in 5 of 1,044 (0.5%) patients.29 A combination of penile ecchymosis or haematoma, sudden penile detumescence, and/or a penile ‘popping’ sound or sensation were reported; in these cases, a diagnosis of corporal rupture could not be excluded. It has been recommended that patients should avoid intercourse for ≥2 weeks after an intralesional CCh injection.26,27 Because of these side effects, clinicians should inform patients with PD prior to beginning treatment with intralesional CCh regarding the potential occurrence of known side effects.26,27 Intralesional CCh remains the best-studied intervention for PD and is currently the only pharmaceutical intervention that has been FDA-approved.
Intralesional interferon-α2b
Interferons are endogenously produced cytokines that are responsible for regulating the immune response to antigenic insults.30 Interferon-alpha2b (IFN-α2b) is thought to improve curvature and reduce plaque size in PD by decreasing the rates of fibroblast proliferation and collagen synthesis.31 Recent studies have suggested that IFN-α2b leads to an improvement in penile haemodynamics, supporting improved erectile function.32
IFN-α2b is a reasonable alternative to CCh as an intralesional treatment, with modest efficacy and an overall excellent safety profile. Intralesional IFN-α2b for ventral penile plaque has similar outcomes and no increased rate of complications compared to dorsal plaques.33
The side effects include myalgias, arthralgia, sinusitis, fever, and flu-like symptoms. They can be effectively treated with non-steroidal anti-inflammatory drugs before interferon injection.34
Further studies are needed to better compare its safety and efficacy compared to other treatments and to assess its functional significance for patients.
Intralesional verapamil
In vitro calcium channel blockers (CCBs), such as verapamil, have been shown to increase collagenase activity and decrease fibroblast proliferation.30 Reports on the efficacy of intralesional verapamil in PD are varying. In particular, intralesional verapamil improved penile curvature and subjective PD symptoms, particularly in younger patients, without causing any major complications.35
Overall, these findings suggested that intralesional verapamil injections could be advocated for the treatment of non-calcified acute phase or chronic plaques to stabilise disease progression or possibly reduce penile deformity, although large scale, placebo-controlled trials have not yet been conducted.36
Intralesional corticosteroids
Intralesional corticosteroids were first used for the treatment of PD in the 1950s.1 As unfavourable side effects, including local tissue atrophy and fibrosis, made any subsequent surgical interventions more difficult,30 steroid injections are no longer recommended for the treatment of PD.8
Intralesional hyaluronic acid
Hyaluronic acid (HA) is a glycosaminoglycan that has been shown to regulate the immune system by decreasing inflammatory cytokines and thus has been used in multiple medical fields to reduce inflammation and scar formation.37 HA is a promising novel therapy for PD that appears to have some efficacy in improving PD symptoms, but data comparing HA treatment to placebo or alternative therapies are lacking.38 Further prospective randomised clinical trials (RCTs) will need to be performed prior to the routine recommendation of HA.
Intralesional botulinum toxin
Botulinum toxin is used in a number of medical fields to reduce fibrosis and scarring. With this in mind, one study evaluated botulinum toxin type A as a treatment for PD.39 However, more safety and efficacy data from larger trials are required prior to routine usage.
Topical Treatment
Although a topical treatment approach to PD is appealing to patients for reasons of comfort and accessibility, in practice the results are less than ideal.
Topical verapamil
There is no evidence that topical treatments applied to the penile shaft result in adequate levels of the active compound within the tunica albuginea. However, in the largest RCT to date, there was statistically significant improvement in curvature and plaque size in patients receiving topical applications of verapamil (15% twice daily) over the course of 3 months.40 The effect was statistically superior to that of a placebo, and patients who continued the topical therapy after the 3-month trial continued to show improvement throughout the 9 months they were followed. Further studies are needed to reconcile these contradictory findings.
Iontophoresis
Iontophoresis, or transdermal electromotive drug administration, is a method theorised to provide superior tissue penetration for the transdermal application of medications. An unblinded RCT of 60 patients suggested that verapamil administered with iontophoresis had better results than verapamil administered intralesionally.41 Again, further large-scale studies are needed to validate these findings.
Non-Pharmacological Treatment
Penile traction devices
Penile traction devices (PTDs) have been studied as a treatment for straightening the penile curvature in men with PD.42 Some studies have shown ≤25° reduction in curvature, an improvement in sexual function, and a significantly lower risk of surgical intervention.43 It is likely that PTDs will play a more important role in the future as part of combination therapy for early-stage PD. There are no serious adverse events, including skin changes, ulcerations, hypoesthesia, or diminished rigidity. Further studies are needed to define the role of PTDs in treating PD.
Vacuum devices
The application of vacuum devices follows the same principles as traction devices with the drawback of being non-continuous and therapy precluding remodelling of the plaque.
Extracorporeal shock wave therapy
Extracorporeal shockwave therapy (ESWT) has been utilised as a treatment for PD, particularly with the goal of reducing pain.44 Overall, the researchers found ESWT effective for significant pain but not for deviation, plaque size, or sexual function.45 ESWT cannot be recommended as a treatment for PD, according to German researchers.46
Radiotherapy
According to the American Urological Association (AUA), given the potential risks of exposing patients to radiotherapy (RT) in the context of unproven benefits, the panel interpreted these data to mean that RT should not be offered to patients with PD.8
Surgical Treatment
Surgical management of PD is indicated for patients with a deformity that impairs sexual function, such as severe penile curvature, penile instability due to an ‘hourglass’ deformity, or other narrowing deformities. Patients who have failed minimally invasive therapy, have underlying refractory ED, have stable disease, or who desire rapid and reliable results may be surgical candidates as well.47 Surgery is indicated when PD is stable for ≥3 months (without pain or deformity deterioration), which is usually the case after 12 months from the onset of symptoms, and intercourse is compromised due to deformity. The goal of surgical treatment is to straighten the penile curvature deformity, preserve or restore erectile function, and preserve penile length and girth.48,49
Penile shortening is present in almost all patients with PD. Thus, it is important to document the penile length prior to surgical intervention so patients realise that the length loss postoperatively is mainly the result of the PD and not the surgery.50
Plication
Penile plication is an option for patients with penile curvature ≤60° and adequate erectile function, either with or without the use of erectile aids. Operative techniques for penile plication surgery have evolved over time. However, a lot of different modifications have been described and the level of evidence is not sufficient to recommend one method over another.51
Penile plication is the most commonly offered surgical intervention to patients with PD, and has a success rate of 82–90% for the resolution of penile curvature.52 The most common complications of surgery are pain and penile haematoma or swelling. Importantly, the final length of the penis following this procedure is equivalent to the length of the shorter side of the penis prior to plication.
Plaque incision or excision with grafting
Graft incision and grafting is indicated for the patient with a complex penile curvature deformity >60°, a large size plaque, a destabilised ‘hourglass’ or hinge configuration, or a short penile length who can achieve adequate rigidity with or without pharmacologics.47,53 A number of grafts have been used, including autologous (derived from the individual’s own body), allografts (derived from a donor of the same species), and xenografts (derived from different species) (Table 2). More recently, the use of buccal mucosa grafts has been advocated. Buccal mucosa grafts provided excellent short-term results, suggested by the fast return of spontaneous erections and prevented shrinkage, which is the main cause of graft failure. It also proved to be safe and reproducible, thus representing a valuable treatment option for PD.54 Due to concerns of the risk of infection and fibrosis, synthetic grafts are not recommended.48 Success and satisfaction rates vary with the different procedures. A RCT evaluating the surgical outcomes between 61 patients undergoing penile plication and 81 patients undergoing plaque excision wıth grafting found outcomes of penile rigidity, penile length, penile sensation, and the ability to achieve orgasm to be comparable between the two groups.55 Overall, 82% of plication patients and 75% of plaque excision with grafting patients reported being very satisfied or satisfied postoperatively.55
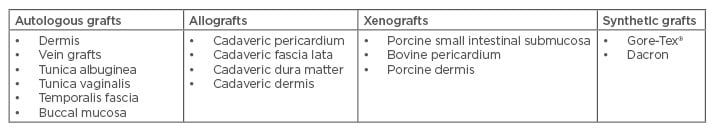
Table 2: Types of grafts used in Peyronie’s disease surgery.
Most recently, Hatzichristodoulou et al.56 described the feasibility and efficacy of collagen fleece as a promising new graft material. Grafting was performed by a ready-to-use collagen fleece coated with tissue sealant, following partial plaque excision/incision.56 In that study, total straightness was achieved in 83.6% of patients. Three patients required surgical drainage because of subcutaneous haematoma formation. Glans sensation was normal in 56 patients (91.8%). Major advantages are decreased operative times and easy application. However, long-term clinical outcomes are necessary to confirm these encouraging findings.
The ideal graft has not yet been found. Surgical experience, along with erectile function, penile length, degree of curvature, and patient preference, all remain important variables in terms of graft selection.57
Penile prosthesis implantation
Placement of an inflatable penile prosthesis is indicated for a patient with PD who has pharmacologic therapy-refractory ED. Approximately 30% of patients presenting with PD have concomitant ED. In addition to placement of the prosthesis, further intervention may be needed to straighten the penis. One study noted that inflatable penile prostheses are associated with greater functional satisfaction and lower rates of persistent penile curvature deformity compared to malleable prostheses.47 Guidelines for placement of penile prostheses for PD are available.58 Intraoperative penile modelling is indicated if a penile curvature deformity >30° remains after prosthetic placement. Intra-operative ‘modelling’ of the penis over the inflated cylinders manually bent on the opposite side of the curvature for 90 seconds, often accompanied by an audible crack, has been introduced as an effective treatment.59,60 If there is a residual curvature of <30°, no further treatment is recommended, as the prosthesis will act as a tissue expander and will result in complete correction of curvature in a few months.59 If a residual curve >30° remains after modelling, then various techniques, including plaque releasing incision, are the next step. Grafting can be considered if tunical defects are >2.0 cm.61,62
Success rates with penile prosthesis implantation for PD range from 84–100%.58,63,64 Partner satisfaction after placement of a penile prosthesis for PD was reported at 60.0–88.8%.63 Complications associated with penile prosthesis include infection, loss of penile length, decreased penile sensitivity, malfunction of the prosthesis, erosion of the prosthesis, and persistent curvature.48
CONCLUSION
PD is estimated to affect 3–9% of men in the general population and is associated with significant negative effects on physical and psychosocial wellbeing, and quality of life. A variety of therapies have been used to treat PD with little available evidence to support their usage. Over the past 30 years, multiple modalities for the treatment of PD have come into view, and most have quietly disappeared. The early recognition of PD patients with their disease-specific issues will allow physicians to select the optimal treatment approach for their patients. More robust data from pooled analyses or databases at multiple high-volume centres would allow for a better retrospective review of various surgical and non-surgical outcomes.








