Abstract
Metabolic syndrome (MS) is a highly prevalent disease related to the risk of cardiovascular disease and diabetes. A large body of evidence has suggested a link between MS and the components of MS with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH) complex. The pathogenesis of MS is complex and not fully understood. Furthermore, recent results from epidemiological studies, including multiple Asian reports, have not been consistent. The risk of BPH is lower in Asian men compared with white men and the prevalence of MS varies by race and ethnicity. An elevated risk of Type 2 diabetes mellitus, hypertension, and dyslipidaemia is closely related to MS and is observed in Asian men even if their body mass index is low. However, the role of race and ethnic disparity in the link between MS and LUTS secondary to BPH is not elucidated. It has been suggested that the pathogenesis of LUTS is multifactorial rather than developing from BPH, which is the traditional concept. Lifestyle and genetic factors may substantially modify the risk of MS and LUTS/BPH. This comprehensive literature review summarises the scientific evidence of the racial/ethnic disparity regarding the association between MS and LUTS/BPH in order to improve current understanding of this controversial issue.
INTRODUCTION
A cluster of multiple risk factors including obesity, insulin resistance, hypertension, and dyslipidaemia that are related to an increased risk of cardiovascular disease has been termed metabolic syndrome (MS).1 The MS and benign prostatic hyperplasia (BPH) complexes are prevalent diseases that increase with age in older men. Hammarsten and Högstedt2 first suggested that components of MS constituted risk factors for the development of BPH. Clinical and epidemiological evidence have suggested that the risk of lower urinary tract symptoms (LUTS)/BPH is associated with MS, or individual components of MS such as obesity, diabetes, and hypertension.3,4 Conversely, other studies, including multiple Asian epidemiologic investigations, did not demonstrate a link between MS and LUTS.5-7
In a multi-ethnic study in the USA, the estimated prevalence of MS was about half as likely in non-Hispanic black males when compared with non-Hispanic white males.8 Race and socioeconomic status are independently associated with BPH. The severity of LUTS is greater in American black men than American white men.9,10 In contrast, no significant difference in the prevalence of symptomatic BPH between these two races of American men was observed in the National Health and Nutrition Examination Survey (NHANES) III.11 Black men are approximately half as likely to be diagnosed with BPH than white men, despite being more likely to undergo surgical treatment.12 In a recent cross-sectional study, multi-ethnic Asian men and Malay men were groups found to be significantly associated with LUTS, particularly post-micturition symptoms among Malay, Chinese, and Indian men.13 The results suggest that the findings can be attributed to religious custom. However, the impact of genetic factors was not clear for this disparity. It is well described that racial variations exist with regard to the age-specific prostate specific antigen (PSA) level, prostate volume, and the prevalence of obesity and diabetes, which are also variables related to the association between MS and LUTS.14-16
There is thus an emerging concern regarding whether race/ethnic differences have a role in the conflicting observations regarding the link between MS and LUTS/BPH. Race/ethnicity reflects any combination of genetic and environmental influence including cultural, lifestyle, and socioeconomic state, both separately and jointly.17 However, little information for racial/ethnic difference with regard to this issue is available. This review provides an overview of the pathway linking variables related to ethnicity with underlying factors between MS and LUTS, with regard to the role of race/ethnicity in their association.
RACIAL DIFFERENCE OF BENIGN PROSTATIC HYPERPLASIA AND SECONDARY LOWER URINARY TRACT SYMPTOMS
Race/ethnicity is associated with the risk of BPH. The risk of BPH has been found to be 41% higher among black and Hispanic men when compared with white men.9 Prostate volume is greater in Caucasian men than in Asian men, consistent with increasing age. In a Japanese study, the mean prostate volume was 20.3 mL in Japanese men and 29.6 mL in American men.15 This observation was confirmed by autopsy.18 The prostate volume was suggested to be related to different cellular composition.19 The prostate volume appears to increase steadily at about 1.6% per year in randomly selected communities of men.20 However, changes in prostate size are highly variable at an individual level. In the Baltimore Longitudinal Study of Aging, at a median follow-up of 4.3 years prostate size had increased in 61.9% of men and remained stable or decreased in 38.1%.21 MS and its components, particularly dyslipidaemia, central obesity, and hyperinsulinaemia, are strongly associated with greater prostate volume increments22,23 and increased risks of symptomatic BPH and LUTS.3,24 The Boston Area Community Health (BACH) survey involving a population-based random sample of a multi-ethnic population found the prevalence of LUTS was consistent according to race/ethnicity.25 Remarkably, an increasing prevalence of LUTS severity is concomitant with ageing, but not with prostate volume, without ethnic disparity.23 In a meta-analysis, although men with MS had a significantly higher total prostate volume than those without MS, the differences between patients with or without MS for LUTS severity was not significant.23
RACE/ETHNIC DIFFERENCE AND OBESITY
The prevalence of obesity varies according to race/ethnic groups, age, and sex.16,26 Strong genetic components in the aetiology of obesity have also been demonstrated.27 Genetic factors may contribute 70% of the variation in body mass index (BMI). Race has been shown to modify the relationship between androgenic steroids and metabolic variables associated with the risk of diabetes. NHANES data has shown that the prevalence of being overweight among men increased significantly while a race/ethnicity disparity remained.28 The highest prevalence of obesity was observed in black men. The prevalence of obesity in men peaked among men aged 40–59 years old at 36.8%.
Asian men possess higher visceral adipose tissue levels than white European men.29 Furthermore metabolic complications of obesity such as insulin resistance, dyslipidaemia, and Type 2 diabetes mellitus have been shown to occur at lower BMI levels among obese Asian men compared with their Caucasian counterparts. It is suggested that with limited superficial subcutaneous fat stores in Asian men, excessive fat deposits more readily in the presence of a positive energy balance.
With a similar BMI, Asian people have a higher cardiovascular risk than Caucasian people, and cardio-metabolic risk is significantly higher in white people than in African Americans.30 A World Health Organization (WHO) expert consultation proposed that the BMI cut-off points, defined by the WHO (25.0–29.9 kg/m2 for overweight, ≥30.0 kg/m2 for obesity) as international classifications, are applicable to Asians.30 The prevalence of obesity is related to ethnic sociocultural differences including diet and physical activity. Specifically, visceral obesity rates in Japanese men are significantly higher than Mongol groups, although the two groups are considered genetically similar. However, the prevalence of individual metabolic abnormalities within each of the groups was similar to each other and also to the reported prevalence rates in the USA.31 Urbanisation and an unhealthy diet may have contributed to an increased prevalence of obesity in Mongolian men. Despite the strong influence of genes on obesity, the non-genetic determinants of obesity and weight change in adulthood, such as dietary behaviour, lifestyle, and physical activity, play an important role in the development of the condition.32
Racial Difference and Diabetes
The prevalence of diabetes was higher in Asia than in the USA.33,34 The prevalence of Type 2 diabetes mellitus has rapidly increased in native and migrant Asian populations and it is very high among Pima Indians. Three developing Asian countries: India, China, and Pakistan, make up 21%, 12%, and 5% of these rates, respectively. One of the hallmarks of diabetes in Asian countries is the rapidly increasing prevalence of Type 2 diabetes in young patients.34 The mechanism of the underlying development of diabetes is complex. Hyperinsulinaemia, a characteristic of insulin resistance, is common among Asian people.35 Asian populations are more insulin resistant when BMI is lower than people of many other races. Diabetes develops at least a decade earlier in Asian people than in white people. The rapidly increasing rate of diabetes in Asia may be associated with genetic and environmental interaction, which is influenced by lifestyle and diet changes caused by urbanisation, and the longevity of life.36 Epidemiologic studies have consistently reported diabetes or glucose levels to be associated with an increased risk of LUTS, regardless of BPH.9
RACIAL DIFFERENCE AND METABOLIC SYNDROME
In NHANES III and NHANES 1999–2006, a persistent increase of MS (from 29.1% to 34%, age adjusted) among different races/ethnicity has been observed.26 Mexican-Americans showed the highest prevalence but had the least change in this time. An increase in MS prevalence is likely related to an increase in diabetes prevalence. However, the prevalence of MS among men without diabetes did not significantly change.
Diabetes and obesity are major components of MS. In Caucasian people the prevalence of MS decreased by approximately half when compared with Caucasians with diabetes.37,38 A markedly lower prevalence of MS in Asian ethnic groups was observed. The prevalence of MS in Japan and in Mongolia, according to Adult Treatment Panel (ATP) III criteria, was 6% and 12%, respectively. In contrast, these rates increased by 13% and 52%, respectively, when based on ATP III WPRO (Regional Office for the Western Pacific Region of WHO) criteria.30 A distinct difference between Japanese and Mongolian populations is the metabolic variable of obesity and dyslipidaemia rates. This finding is attributed to a difference in diet and lifestyle.
A consistent epidemiological finding is that the prevalence of MS peaks in people who are middle-aged, followed by its decline or equilibrium in men aged 70 years or older.37,39-41 The trend of a declining prevalence of MS among older-aged men was similar across different races.
The prevalence of MS gradually increased in men from 20 years old up to 50–59 years old according to the Japan Metabolic Syndrome Criteria Study Group guidelines, or up to 60–69 years according to the United States National Cholesterol Education Program (NCEP) guidelines, followed by a decline at 70 years and older.39 The highest prevalence rate of obesity and dyslipidaemia was observed at around 50 years of age, while the prevalence of hypertension increased in both genders from 20 years through to 80 years of age.
In a Korean cohort study, the prevalence of MS showed a peak in those aged 65–74 years old following which it then gradually decreased in older men but increased in older women.41 The prevalence of hypertension and diabetes increased in men aged ≥65 years old. Dyslipidaemia rates remained constant in the older-aged men. In Asian populations, it is considered that an increased prevalence of MS is attributed to an increase in the prevalence of obesity-related diabetes mellitus.
RACIAL DIFFERENCE OF PROSTATE SPECIFIC ANTIGEN LEVELS
Prostate volume and PSA level were shown to be the most powerful predictors of BPH progression.14,42 In a large study, among unselected populations of healthy ageing-men, elevated levels of free PSA independently increased the risk for clinical BPH over 9 years of follow-up.43 In a 15-year longitudinal community-based study of Japanese men, LUTS and quality of life deteriorated, prostate volume and PSA level increased, and maximum flow rate decreased.44 Racial differences in the age-specific PSA reference range is well known. Asian men had lower PSA values than white and African American men of similar age.14,45 In a cross-sectional study in Malaysia among multi-ethnic groups, the median PSA level of Malay, Chinese, and Indian men was 1.00 ng/mL, 1.16 ng/mL, and 0.83 ng/mL, respectively.46 Indians had a relatively lower median PSA level and prostate volume than Malay and Chinese men. Asian American men had the highest PSA level, and the lowest PSA levels were observed in the Chinese men within the Asian men cohort.45 The results indicated that environmental and dietary factors may influence the serum PSA level.
A prospective longitudinal study demonstrated that at the baseline, median PSA level in white men did not differ from the levels in African American men.47 However, African American men had a much more rapid increase in the PSA level over time when compared with white men. This suggests that genetic and epigenetic factors may contribute to racial/ethnic differences in the serum PSA level.
Multifactorial Aetiology of Lower Urinary Tract Symptoms
LUTS is not a definitive diagnosis and is subject to complex symptoms, with LUTS pathologies affecting prostate and bladder function. A traditional concept of LUTS in elderly men refers to bladder outlet obstruction caused by benign prostatic enlargement. This view did not involve the ageing bladder as a possible cause of LUTS. Age and bladder outlet obstruction are independently associated with detrusor overactivity in men with BPH.48 In a prospective cohort study of BPH treatment effectiveness, LUTS was not associated with prostate volume, however a reduction in symptoms following treatment correlated with uroflowmetry results.49
Poor correlation with symptoms suggested that LUTS defined by the International Prostate Symptom Score (IPSS) was an unreliable measurement of pathophysiological phenomena regarding prostatism.
Autonomic nervous system hyperactivity is significantly associated with LUTS.50 The distribution of weak stream frequency was greater in the Japanese than in American men, despite smaller prostates.14 The results support that LUTS are associated with a non-obstructive causal relationship.
LIFESTYLE/MODIFIABLE RISK FACTORS AND LOWER URINARY TRACT SYMPTOMS
Lifestyle interventions such as weight loss, increased physical activity, and healthier dietary habits substantially alter the risks of symptomatic BPH and LUTS.25,43,51 In a meta-analysis, the intensity of exercise was related to the reduction of risk of prostate enlargement.23 High-energy intakes, particularly with a high consumption of protein and polyunsaturated fatty acid, led to a greater risk of developing BPH.52 Interestingly, a protective effect of alcohol intake on BPH/LUTS was consistently observed in various studies.51 The impact of modifiable risk factors on LUTS secondary to BPH seems to be similar across different races. The use of prescribed medication that potentially contributes to LUTS as adverse effects was associated with LUTS.53
DISCUSSION
In an epidemiological study, race/ethnicity may not reflect the genotype if categorisation of race/ethnicity is defined by phenotype or social group. The relationship between individual metabolic components of MS and LUTS/BPH is complex (Figure 1). Basic and clinical investigation demonstrated that metabolic components including obesity, diabetes, and BPH are substantially influenced by genetic and shared environmental factors such as dietary habits and physical activity.54 Although there is a strong genetic influence on the development of obesity and diabetes, the role of ethnic disparity in the link between MS and LUTS may overlap genetic variations, lifestyle, and socioeconomic factors. There are environmentally determined differences across different population groups observed over time. Recently, increases in the prevalence of overweight and obese adolescents and children have been observed in many countries throughout the world.55 This increased trend is attributed to environmental factors. The emerging high prevalence of obesity in Asia is based on factors other than ethnicity such as urbanisation, nutrition transition, and socioeconomic status.
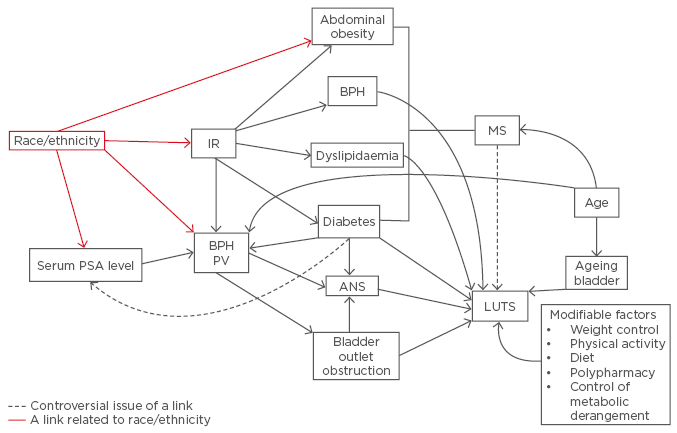
Figure 1: The association between variables related to ethnicity and underlying factors relating to metabolic syndrome and lower urinary tract syndrome.
IR: insulin resistance; MS: metabolic syndrome; PV: prostate volume; ANS: autonomic nervous system; BPH: benign prostatic hyperplasia. PSA: prostate specific antigen; LUTS: lower urinary tract benign prostatic hyperplasia.
Epidemiological data from the USA and Asia suggest that despite the unrelenting increase in the prevalence of obesity, rates of MS and obesity are dependent on age, through middle-age when these rates peak and begin to either decrease or remain constant in older-age across different racial groups. It is remarkable that the prevalence of overweightness among children and adolescents along with obesity among men increased significantly among different races.55 Trends did not differ significantly by age or racial/ethnic group. In a Korean community-based study the rate of MS decreased among men aged ≥70 years old. The prevalence of obesity plateaued at 36.2% in 2007 and 37.8% in 2012. Age-specific prevalence of hypertension and diabetes remained constant, while the prevalence of obesity and dyslipidaemia decreased in old-age (>70 years). This finding may be linked to increased rates of regular heath examinations which reached >70% in old age males. These results might be attributable to lifestyle changes and better control of blood glucose and blood pressure in accordance with improved socioeconomic status in older age. In Korea, regular heath examination rates substantially reached >70% in older males (≥65 years old).41
In Asian populations the prevalence of diabetes has greatly increased. The risk of central obesity in Asian populations is higher due to genetic influences. The rapidly increasing rate of diabetes in Asia is associated with genetic factors and environmental interaction. Epidemiological studies have demonstrated that the environmental impact on the risk factors of diabetes may be similar across different races.
The correlation of diabetes to prostate volume is not clear. Unexpectedly, in the BACH survey when controlling for age, men with diabetes had a 21.6% lower mean PSA level than men without diabetes.56 It is suggested that serum testosterone levels and diabetes medication are associated with a decrease in PSA level. A multi-ethnic community-based study showed that the presence of diabetes may be less related to prostate volume, but more so to LUTS, irritative symptoms, and nocturia.47
LUTS are strongly dependent on age and the presence of MS. The possible mechanism of the link between MS and LUTS is that of hyperinsulinaemia, which is an underlying feature of MS and is causally related to the development of BPH by an increased sympathetic nerve activity in men with BPH.2 However, conflicting results were observed in linking LUTS to prostate volume. The amount that prostate volume increases with advancing age is highly variable between different individuals.21 Development of BPH is traditionally related to genetic predisposition which is an unmodifiable factor. MS and its components are associated with a greater prostate volume.22,23 Increased physical activity and dietary changes modify the risk factors of BPH/LUTS.
As defined by the IPSS, LUTS presents dynamic aspects over time across a person’s life.21 In a 3-year follow-up of Japanese men, LUTS worsened in approximately one-third of the participants, remained the same in one-third, and improved in one-third.15 In a USA longitudinal study, at a 4.2-year follow-up, about 20% of men in the community showed an increase of IPSS points and about 16–23% had an increase in prostate volume.57 Age-specific IPSS is higher in Japanese men than American men despite a lower prostate volume than American men.58 The prevalence of LUTS is variable with age; the prevalence of moderate-to-severe LUTS ranges from 22% in men aged 50–59 years old, to 45% in men aged 70–80 years old. Inconsistent results in the link between MS and LUTS may be attributed to the interaction of hereditary and environmental factors. The dynamic nature of individual metabolic components impacts the association between MS and LUTS.
CONCLUSION
In epidemiological investigations, racial and ethnic categories are social and not genetic. The inconsistent results concerning the link between MS and LUTS reflects the diversity in genetic and socioenvironmental factors associated with it. Although there is a strong influence from genetic impacts on obesity and diabetes, the role of ethnic disparity in the link between MS and LUTS may overlap genetic variations, lifestyle, and socioeconomic factors. A tangled web of relationships between LUTS and metabolic variables may hamper the impact of race/ethnicity on the link between LUTS and MS. Emerging new concepts on pathophysiology of LUTS and lifestyle factors may provide new targets for modifying the risk factors of MS. Longitudinal studies are needed for a better understanding of the relationship between MS and LUTS.