Abstract
This scoping review emphasises the significance of various complications such as urinary tract infections (UTI), urethral pain, urethral trauma, damage, and haematuria, when understanding and reporting complications experienced during intermittent catheterisation using hydrophilic-coated and newer catheters with integrated hydrophilic properties.
Currently, there is a lack of consistency and interchangeability in the definitions of bladder and urethral complications in published literature, particularly in the case of urethral trauma and damage.
A search of the literature was conducted in accordance with established Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA) guidelines. By mapping the terminology used in articles, and providing insight into the current gap in the definition of complications related to catheter use, the authors highlight the urgent need for standardisation in the definitions of complications associated with catheter use. This will enhance research and education, and better manage and address intermittent catheter-associated complications (ICAC), promoting best practices in catheter education, use, and selection.
INTRODUCTION
Catheters have been utilised since ancient times to assist individuals incapable of voluntarily emptying their bladder. Clean intermittent self-catheterisation became prominent in the 1970’s giving individuals more autonomy to manage this procedure themselves.1,2 Presently, intermittent catheterisation (IC) is considered the gold-standard for neurogenic lower urinary tract dysfunction in those with good hand function, or a willing caregiver. 3-5
In a roundtable discussion in April 2023,6 the authors focused on ICACs, and identified several complications linked to intermittent catheter use.1-5 The discussion also highlighted limitations in previous studies, including the lack of detailed baseline information about the study populations, and that the outcomes related to intermittent catheterisation use were not sufficiently aligned with urethral complications.7 This therefore underscores the challenge of establishing true incidence and prevalence data for each complication.
More well-structured clinical trials are needed to provide a detailed analysis of adverse events related to intermittent catheterisation.
This scoping review aims to systematically map the terminology used in articles that evaluate ‘hydrophilic’ intermittent catheters, including catheters with a hydrophilic coating, or newer devices with hydrophilic properties incorporated into the catheter material, such as the integrated amphiphilic surfactant, with a particular focus on terms related to ICACs.
It should be noted that the focus of this scoping paper is on hydrophilic intermittent catheter complications, and it is not an in-depth review on the many aspects of catheter-associated urinary tract infections. A recommendation for a comprehensive definition of the symptoms of catheter-associated urinary tract infection (CAUTI) is outside the scope of this review.
METHODS
This review adheres to PRISMA.8 The literature search strategy was focused on PubMed, Cochrane Library, Embase, and Google Scholar, containing the following combination of terms: “urethral AND (hydrophilic OR coated OR integrated amphiphilic surfactant) AND (intermittent AND [catheterisation OR catheterization OR catheter OR dilatation]) AND (pain OR discomfort OR sticking OR stickiness OR haematuria OR hematuria OR bleeding OR urethral trauma OR trauma OR obstruction OR neurological urinary tract dysfunction OR retention OR bladder outlet obstruction OR false passage OR stricture OR stenosis OR scarring).”
Literature was limited to human studies published from 1990–October 2022, and written in English. Two reviewers screened all identified literature, and evaluated the titles, abstract, and full text. In instances of disagreements, resolution was sought through consultation with a third reviewer.
RESULTS
Selection of Data
The systematic search identified a total of 400 articles, of which 41 were evaluated: 17 original articles16-32 and 24 review/meta-analysis articles (Figure 1).11,13-15,33-52 Additionally, a hand search identified seven more original articles.53-59 A total of 48 articles were analysed. This illustrates the search strategy, and reflects the quality of the literature base.
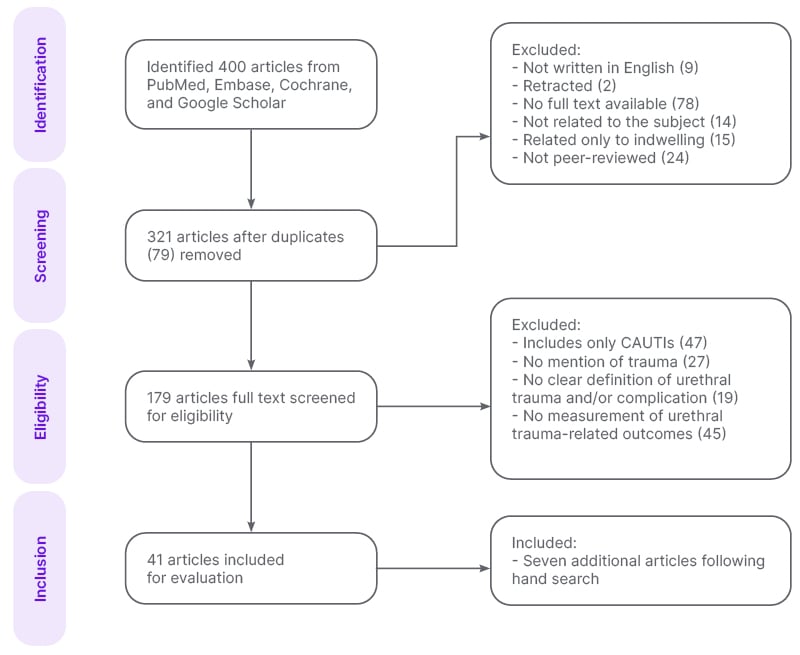
Figure 1: Schematic of the systematic search across PubMed, Embase, Cochrane Library, and Google Scholar to select articles for analysis.
CAUTI: catheter-associated urinary tract infection.
GENERAL DEFINITIONS OF URETHRAL COMPLICATIONS/TRAUMA
This scoping review identified three main categories when reviewing the literature for ICACs: definitions of urethral complications; complications in association with defining urethral trauma; and definition of intermittent catheterisation complications.
Not only were there different categories discussing intermittent catheterisation complications, but there was a wide variety of descriptions of these complications within each of these categories. This highlights the challenges and needs for this scoping review to unpack the significant overlap between these categories.
Definitions of Urethral Complications
In the existing literature, the term ‘urethral complications’ is frequently used to describe the side effects associated with IC, as evidenced by numerous studies.17,18,23,27,28,32-35,37,40,43-45,47-50,55,56,59 However, this term often lacks a precise definition, and is typically represented with considerable variability (Supplementary Table 1). The complications range from paired complications, e.g., ‘UTIs and haematuria’,32 and ‘strictures and false passages’,18 to extended lists of multiple complications, such as ‘urethral false passage, urethral strictures, gross haematuria, and recurrent UTIs’27 among others. This lack of specificity in definition across various articles underscores the need for a more standardised description of these complications.
The range of complications associated with IC mentioned in the literature varies widely, from one to as many as 20 different types, with UTIs often identified as the most common complication.35,58 In terms of multiple complications, four articles specifically discuss dual issues: for instance, UTIs coupled with urethral trauma,31 or UTIs coupled with haematuria,32 each pair representing a separate outcome. Notably, Hakansson et al.30 report a high incidence of UTIs in 77% of IC users,59 and urethral trauma in 30%.61
In contrast, some sources focus their definitions of complications specifically on outcomes induced by trauma, excluding UTIs. For example, one study40 highlights complications such as bleeding, urethral stricture, and false passage. Similarly, another source50 details complications like strictures, false passages, urethritis, and other conditions stemming from urethral trauma.
More comprehensive definitions of complications associated with IC are found in other publications,45,47 specifically eight distinct complications, including ‘UTI, urethral stricture, haematuria, bladder stones, false passage, pain or discomfort, and upper tract complication of renal scarring’ (Supplementary Table 1). Others, such as Prieto,47 categorise complications into two distinct groups: one that considers UTIs, and another that does not.
At the other end of the spectrum, definitions of IC-related urethral complications vary widely. For instance, Kanti et al.48 documents as many as 14 different complications, while another36 lists up to 20. The approach taken in Kanti et al.48 presents a comprehensive range of catheterisation complications, which are not categorised by catheter type, suggesting an extensive coverage of complications induced by IC.
The most elaborate description of urethral complications is found in Li et al.,38 which identifies 20 complications associated with catheterisation. These are further categorised into various groups, including hypersensitivity-related issues, catheter blockage, and malignancy, among others. While this source examines outcomes from all types of catheterisations, not exclusively IC, their comprehensive definitions, along with those from other sources, are systematically compiled in Supplementary Table 1.
Additionally, in one instance,33 the phrase ‘urethral problems’ is used, encompassing a range of issues such as discomfort, blockage, infection, haematuria, trauma to the urethra, prostate, or bladder, and expulsion. This description summarises the variety of complications mentioned in Supplementary Table 1.
Key Points on the Definitions of Urethral Complications
- Broad versus specific: some studies provide broad definitions that include a wide range of complications; others offer more specific lists, focusing on complications like strictures, false passages, and haematuria.
- Inconsistency in classification: the definitions vary significantly from one study to another, with some categorising complications as acute or long-term, and others not making such distinctions.
- Frequency and severity: some definitions highlight the frequency of certain complications, such as urethral bleeding, while others mention the severity of the challenges these complications pose.
- Differing emphasis: studies also differ in their emphasis on complications. While UTIs are commonly mentioned, some definitions emphasise the mechanical trauma induced by catheterisation, and others highlight less common complications, such as catheter blockage and urinary tract malignancy.
Complications in Association with Defining Urethral Trauma
In numerous instances, the side effects of IC have been commonly characterised as ‘urethral complications’. Among the 48 articles that provided a definition or explanation regarding urethral trauma or urethral complications, 20 mentioned UTIs.
UTIs were frequently segregated into a distinct category from other urethral complications, notably urethral trauma.
Subsequently, a count of articles incorporating various specific complication outcomes was undertaken, with a particular emphasis on those independent of UTIs. Urethral bleeding, which encompasses both gross and microscopic haematuria, was cited in 16 articles,13,23,27,28,32,35,37,40,43,45,47,49,53,55,57,59 and stricture was noted in 14 articles13,18,27,37,39,40,43,45,47,49,50,53,55,59 as a definition of urethral complications. Inflammation of the urethra, alternately referred to as urethritis, emerged as the third most frequently mentioned urethral complication, cited on 11 occasions. Additionally, false passage and epididymitis were included in the definitions provided in eight18,27,40,45,47,50,55,57 and four37,43,47 articles, respectively. Other outcomes forming part of the urethral complication definition reported less frequently were related to patient experience (e.g., sticking, pain), UTI-related outcomes (e.g., bladder stones, encrustation, urosepsis), and allergic reactions.
Key Points on the Definitions of Urethral Trauma
- Severity: definitions of urethral trauma (Supplementary Table 2) range from irritation to severe conditions.
- Assessment methods: there is a lack of a standardised indirect and direct method for assessing urethral trauma. Previously suggested techniques include measurement of withdrawal friction-force of catheters, urethral cell counts, urethral cytology, and the presence of haematuria as possibilities,30 but to date none have been universally accepted.
- Associated risks: the sequelae of urethral trauma are frequently mentioned, with a particular emphasis on the increased risk of UTIs, and the potential for long-term complications like strictures.
- Symptoms and indicators: symptoms like pain and bleeding are commonly used to describe urethral trauma, while the presence of haematuria is often used as an indicator of trauma severity.
- Long-term impact: the long-term impacts of urethral trauma, such as stricture formation, and its effect on the quality of life and bladder management, are acknowledged as significant concerns in the definitions provided.
Definition and Description of the Frequency of Intermittent Catheterisation Complications
A total of 48 articles in those who used intermittent catheterisation for their bladder management were scrutinised for their description of complications. The review revealed eight types of complications affiliated with intermittent catheterisation and urethral trauma. The complications considered in these articles were independent of CAUTIs. The complications identified were haematuria, microhaematuria, stricture, false passage, epididymal-orchitis, urethritis, and urethral irritation.
Liao et al.52 defined haematuria, noted for its various terminology including ‘urethral bleeding’ and ‘gross haematuria’, as visible blood presence in the urine. Out of the articles analysed, 13 omitted this complication, 31 cited or measured haematuria with no additional explication, and seven offered a definition or a broad explanation of the term.13,24,37,38,41,51,52 Two among them noted the utilisation of dipstick analysis for haematuria measurement.24,38 Various causes of haematuria were speculated, ranging from urethral trauma50 to a result of CAUTI36 (Table 1).
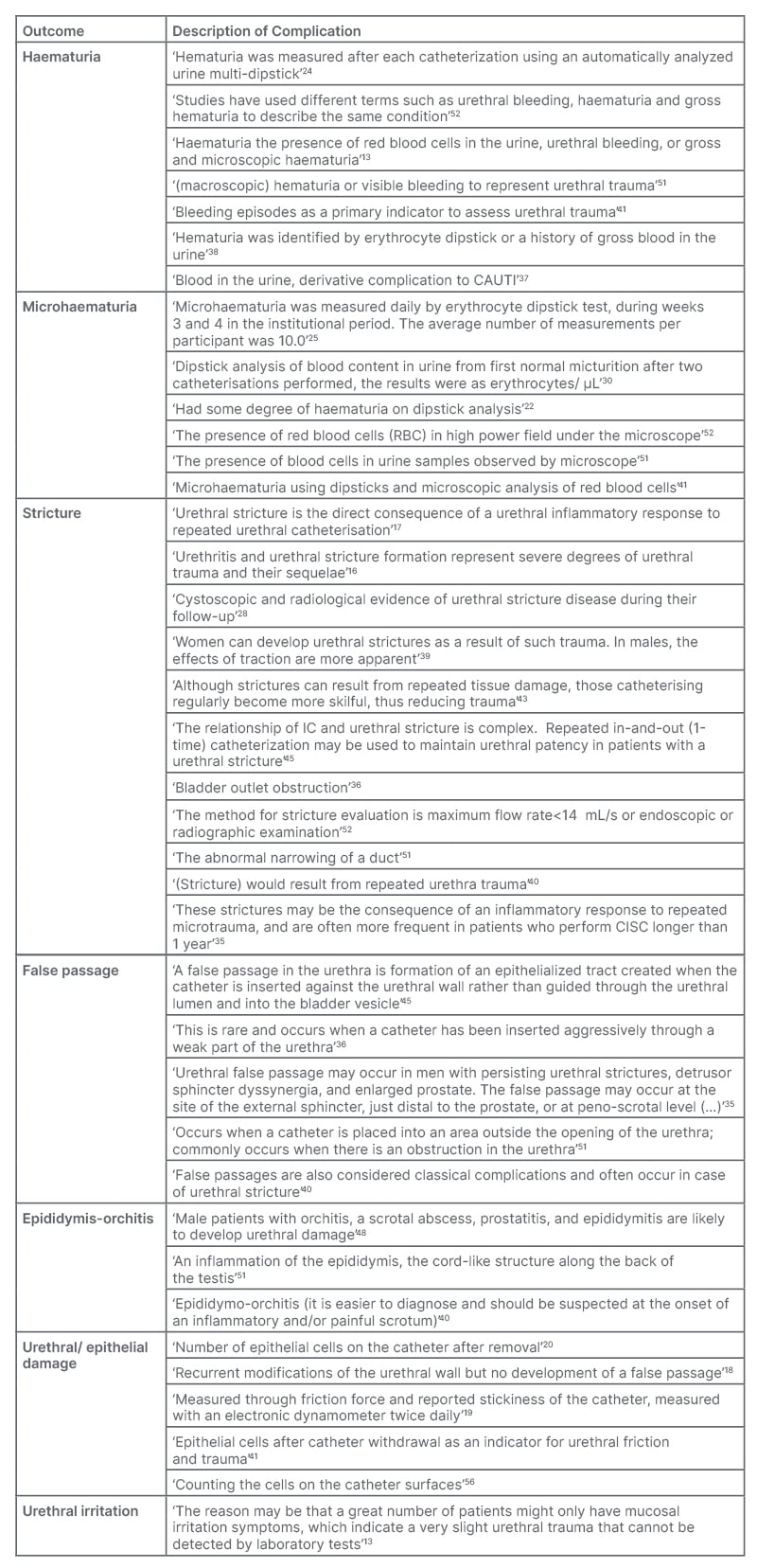
Table 1: Detailed definitions of complications associated with intermittent catheterisation as identified in the analysed articles.
CAUTI: catheter-associated urinary tract infection.
Conversely, microhaematuria is associated with haematuria, but is only observable microscopically.51,52 Of the 48 articles, 35 did not mention this complication, nine mentioned it without elaboration, and six gave measurement methods or provided an explanation.22,25,30,41,51,52 Detection methodologies, according to the literature, incorporate dipstick usage,22,25,30 or microscopic observation of urine for red blood cells.51,52 Occasionally, both methods are employed41 (Table 1).
Urethral strictures emerged as the second most reported traumatic side effect of IC. From the selected 48 articles, 17 omitted this complication, 22 mentioned it, and 11 gave an explanation/definition.16,17,28,35,36,39,40,43,45,51,52 Some definitions were brief,36,51 and others detailed, e.g., ‘The method for stricture evaluation is a maximum flow rate <14 mL/s or endoscopic or radiographic examination’.52 Repeated catheterisation causing urethral damage is cited as a typical cause for stricture formation, leading to inflammation,16,17,35,40,43 with one article38 noting sex-specific causative variations (Table 1).
In relation to intermittent catheterisation urethral trauma-related complications, false passage, or ‘via falsa’, was the third most common. Of the articles, 19 mentioned it without additional explanation, five provided explanations/definitions,35,36,40,45,51 and the rest did not measure or analyse this complication. Engberg et al.45 articulated a clear definition: ‘A false passage in the urethra is the formation of an epithelialised tract created when the catheter is inserted against the urethral wall rather than guided through the urethral lumen and into the bladder vesicle’.45 Two articles considered the outcome a consequence of urethral stricture35,40 or forceful catheter insertion36 (Table 1).
Epididymitis and epididymo-orchitis are well-characterised in the medical realm, and were mentioned in 18 articles, with three providing descriptions.40,48,51 A precise definition was supplied by Ontario:51 ‘An inflammation of the epididymis, the cord-like structure along the back of the testis’. Another article implied that epididymo-orchitis might increase the risk of urethral damage.48 All three definitions/explanations are tabulated in Table 1.
The term ‘epithelial damage’ or ‘urethral damage’ was mentioned in 10 articles, and defined in five articles with explanations.18-20,41,56 The number of epithelial cells after catheter removal seems to be an indicator of damage to the urethra,41,56 or the friction force exerted by the catheter.19 The damage is not followed by the formation of false passage (Table 1).18
Urethral irritation emerged as the least common complication in the context of urethral trauma, secondary to IC. Out of the articles, 11 measured this complication, but only two provided descriptions.11,16 Secretariat11 offered a basic definition, while Vaidyanathan et al.16 stated that this outcome was ascertained through urethral cytology. Urethral irritation, particularly as caused by IC, is frequently cited in review articles. A singular original research article, Ye et al.13 included elucidations regarding this outcome, highlighting it as a distinct indicator of urethral trauma. The term ‘urethral irritation’ surfaced in six articles,13,34,37-39,49 none of which extended further explanations. Comprehensive definitions and descriptions of urethral inflammation and irritation are available in Table 1.
EVOLUTION OF COMPLICATIONS IN URETHRAL TRAUMA RESEARCH
The complications mentioned in the literature have evolved. From 1990–2010, most articles referred to various urethral trauma complications, albeit with scarce provision of definitions. The most mentioned complications throughout the publication history were ‘urethral complication’, ‘urethral trauma’, and ‘epithelial damage’. There are just as many publications between 2011–2020 compared to 1990–2010, and an increase in the number of complications with accompanying definitions. There was, however, the increase in reference to ‘false passage’, ‘haematuria’, ‘stricture’, and ‘epididymo-orchitis’, as examples. However, more can and must be done to simplify and clearly define the frequency and description of complications associated with IC.
In an analysis of original articles, when considering the frequency of complications, and their descriptions in relation to the study duration, the short-term studies of 1–10 days typically tracked one complication. In contrast, studies with durations of 1–6 months tended to investigate multiple types of complications. Notably, no single study covered as many as six types of complications,16 contrary to what was previously mentioned. Studies of 1 year duration typically reported two or three complications, indicating a varied approach to data measurement. Long-term studies, particularly those extending beyond 5 years, examined up to four different complications. This suggests an increasing complexity in outcome measurement correlating with longer study durations.
DISCUSSION
A key finding of this scoping review confirms that there is no unified definition of IC complications within the literature, but rather demonstrates the variation in definitions used. Considering this, the authors support the proposal made during a recent expert roundtable discussion, that to promote clarity and consistency, in both evidence and clinical practice, there is a need to establish a consensus definition for ICACs and associated endpoints. This should not only include symptoms or complications that can be observed and measured, but also consider the individual’s perceived/experienced symptoms that may not always manifest with a physical sign that can be measured.
The term ‘urethral trauma induced by IC’ is also unclear. While several studies attempted to define this term, significant differences exist between them, e.g., “urethral injury can result from improper, difficult, or traumatic, repeated catheterisation,”29 and “IC can cause bruising and trauma to the urethral mucosa.”36 Urethral trauma can refer to the friction force applied by the catheter during insertion or removal, damage to the lining of the urethra, or haematuria. Additionally, most definitions of urethral trauma do not include the type of catheter, the catheter material, or the handling of the catheter as a contributing factor to the trauma. As such, a more uniform and standardised definition of urethral trauma is needed.
It is important to note that most catheterisation trauma-related complications were reported in study durations of 1–6 months, or up to 1 year duration. Furthermore, the extent of traumatic damage to the urethral tissue lining caused by catheterisation is not well-documented.
Several questionnaires focused on patients’ experience but did not address the full range of long-term catheter complications that may provide a better understanding of their connection with complications (e.g., result from the catheter material, high friction force, improper handling of the catheter, etc). Further research is required to establish the mechanistic cause of the pathophysiology of catheter-induced urethral trauma, particularly in light of improvements with newer catheter materials, e.g., integrated amphiphilic surfactant.66
LIMITATIONS
The authors acknowledge a limitation in their search strategy, in that the focus was only on hydrophilic catheters. This exclusion may have narrowed the scope of their findings, as different materials and coatings could influence complication rates and patient experiences. The impact of catheter composition warrants further investigation, and should be considered in future research.
The authors’ review did not address potential variations in complication rates or patient experiences between individuals based on gender, or with and without perineal sensation; nor did it explore differences among patients who can void voluntarily versus those who cannot, or between individuals who catheterise themselves versus being catheterised by someone else. Although data on these distinctions may be scarce, acknowledging the possibility of divergent outcomes among these patient populations is important for a comprehensive understanding of the field. Future research should investigate whether these factors significantly impact patient outcomes.
This review spans a considerable timeframe, during which the terminology used in the literature may have evolved. For instance, terms like ‘microhaematuria’ might also appear as ‘microscopic haematuria’ or ‘non-visible haematuria’. Although the authors have made efforts to account for this variability, it is possible that not all synonymous terms were captured in their search strategy. This may have affected the comprehensiveness of their search results, and should be considered when interpreting the findings.
CONCLUSION
In conclusion, there is no single, universally accepted definition of urethral trauma associated with catheter use. Definitions vary widely, encompassing a range of symptoms, assessment methods, and potential complications. This diversity in definitions not only reflects the complexity of the complications associated with intermittent catheterisation, but also underscores the need for a standardised approach for defining, measuring, and reporting complication outcomes, to improve patient outcomes and standards of care.
With the emergence of new catheter technologies, it is important for the field of urology to establish unified definitions for these urethral complications to support ongoing research, education for patients and healthcare professionals alike, and clinical practice. The expert roundtable discussed the particular challenge in defining UTI symptoms, as there can be an overlap between symptoms of a UTI and of urethral trauma. This situation poses a challenge for future researchers to address, and the authors call on the international expert communities to include all stakeholders including patients, healthcare professionals, users, and industry, to drive a consensus and support by establishing clear definitions for ICACS, and aligning clinical practice for the treatment of CAUTIs.7
RECOMMENDATIONS
Based on this scoping review and multiple definitions of complications, the authors recommend that researchers or investigators, and healthcare providers be as detailed as possible when describing the specific type of catheter-related complication. This is particularly important when discussing what type of definition is being used regarding UTIs, since there are multiple definitions. Investigators should include all types of complications that occur, rather than focusing on just one specific complication. The literature is also lacking with regard to other variables that may impact intermittent catheterisation complications. These variables should include patient-related factors, such as the person’s frequency and compliance at performing catheterisation, whether they are independent when performing catheterisations, gender, pre-existing urethral problems (false passage, stricture), and type of catheter being used. This information would be extremely helpful in improving patient compliance, decreasing complications, and helping to assess current catheters and promote further advances in catheter design.
The authors also recognise the need for validated outcome measures for assessing the patient experience related to IC. Given the importance of patient-centred care, there is a need for the development and validation of tools that accurately capture how much patients feel bothered by the IC process. Researchers should prioritise efforts to develop and validate such measures, to better understand and address patient needs and concerns.







