Nocturia has historically been considered as one aspect of lower urinary tract symptoms (LUTS). However, it has been noted that nocturia has its own multifactorial aetiologies besides LUTS. The relationship between male nocturia and brain natriuretic peptide has previously been shown,1 as well as its controlling nutritional status score.2Additionally, the relationship of male nocturia with smoking exposure and chronic kidney disease has also been clarified, the research of which is to be presented at European Association of Urology congress (EAU20).
Recently, a new diagnostic algorithm was proposed, and many causative factors are summarised in the International Continence Society consensus.3
Cardiovascular causes such as hypertension and heart failure constitute one of six sections. Despite this, the association of nocturia with cardiac load has not been fully examined. The cardio-thoracic ratio (CTR) is one of the most easily available assessment tools for evaluating cardiac load. CTR is calculated as the longest diameter of the heart border divided by the internal diameter of the chest on chest radiographs. In this study, the authors investigated the relationship of CTR with male nocturia.
The authors retrospectively evaluated 425 Japanese men whose CTR and other variables were recorded at their initial visit for LUTS between 2014 and 2018 at a single institution. The frequency of nocturnal voiding was collected using the overactive bladder symptom score questionnaire. Severe nocturia was defined as a frequency of nocturnal voiding ≥3 times. The optimal cut-off value of CTR to predict severe nocturia was 51.2%, according to receiver operating characteristic analysis. Multivariable logistic regression analyses were used to assess associations between severe nocturia and clinical parameters, including CTR, age, prostate volume, BMI, use of any medications for LUTS, serum sodium concentration, estimated glomerular filtration rate, and the presence or absence of hypertension, diabetes, insomnia, sleep apnoea syndrome, cardiovascular disease, or cerebrovascular disease.
The median patient age was 72 years. The frequencies of nocturnal voiding were 0, 1, 2, and ≥3 in 28, 86, 92, and 219 men, respectively. The median CTR was significantly higher amongst men with severe nocturia than those without (48.3% versus 47.2%; p=0.013). Multivariable analysis (as shown in Table 1) revealed that a CTR ≥51.2 (odds ratio [OR]: 2.14; p<0.01) was an independent risk factor for severe nocturia, as were higher age (OR: 1.65; p=0.03), lower BMI (OR: 2.10; p<0.01), the presence of sleep apnoea syndrome (OR: 3.46; p=0.03), lower sodium concentration (OR: 2.16; p=0.02), and lower estimated glomerular filtration rate (OR: 2.62; p<0.01).
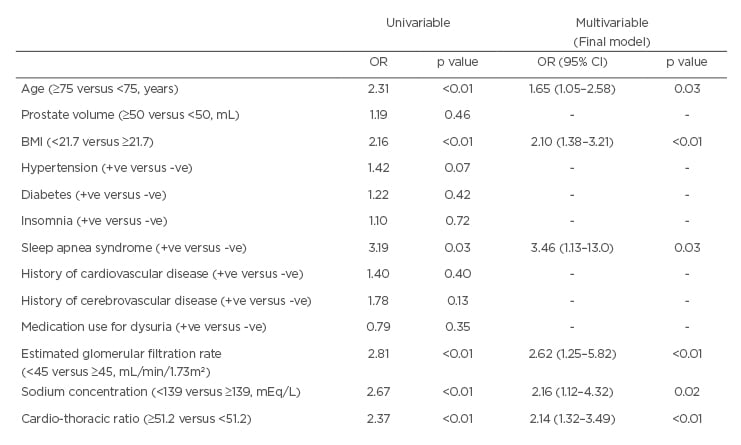
Table 1: Univariable and multivariable analyses of variables relationships of severe nocturia in men.
BMI: body mass index; CI: confidence interval; OR: odds ratio; +ve: positive; -ve: negative.
The relevance of CTR to the severity of male nocturia was demonstrated. CTR, a simple and routinely used assessment tool for cardiac load, may be a biomarker indicating the severity of nocturia associated with cardiac overload. Recently, desmopressin has been often used as a pharmaceutical therapy for nocturia, which requires a careful follow-up to assess its effects on cardiac load. These findings also caution against careless prescription of such medicine for men with nocturia; rather, it may be worth considering diuretics for men with a higher CTR. Future studies should look at whether kinetic changes in CTR reflect the therapeutic effects of diuretics.
In conclusion, CTR is a possible biomarker associated with the severity of male nocturia. Evaluating CTR in daily practice may help clinicians select optimal treatments for nocturia.








