Chairperson: Robert Landewé1,2
Speakers: Fabian Proft,3 Anna Moltó,4 Martin Rudwaleit5
1. Academic Medical Centre, Amsterdam, the Netherlands
2. Zuyderland Medical Centre, Heerlen, the Netherlands
3. Charité – Universitätsmedizin Berlin, Berlin, Germany
4. Hôpital Cochin, Paris, France
5. Department of Internal Medicine and Rheumatology, Klinikum Bielefeld, Bielefeld, Germany
Disclosure: Prof Landewé has received consulting fees, research or institutional support, and educational grants from AbbVie, Bristol-Myers Squibb, Celgene, Galapagos, Gilead, Eli Lilly, Janssen, Merck, Novartis, Pfizer, and UCB; owns Rheumatology Consultancy BV; and is a Past-President of Assessment of SpondyloArthritis international Society (ASAS). Dr Proft has received consulting fees, research or institutional support, and educational grants from AbbVie, Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB. Dr Moltó has received consulting fees, research or institutional support, and educational grants from AbbVie, Bristol-Myers Squibb, Merck, Pfizer, and UCB. Prof Rudwaleit has received consulting fees, research or institutional support, and educational grants from AbbVie, Bristol-Myers Squibb, Celgene, Chugai Pharmaceutical Co., Ltd, Eli Lilly and Company, F. Hoffmann-La Roche AG, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and UCB.
Acknowledgements: Medical writing assistance was provided by Jennifer Taylor, London, UK.
Support: This nonpromotional meeting was organised by UCB.
Citation: EMJ Rheumatol. 2020;7[2]:2-10.
Meeting Summary:
This symposium took place during the European League Against Rheumatism (EULAR) 2020 E-CONGRESS. Focussing on the optimal management of axial spondyloarthritis (axSpA), the faculty discussed sex-specific challenges and approaches in the management of axSpA, evolution in the concept of the disease, and evolving treatment targets and strategies. Dr Proft presented a case illustrating how a patient-based online referral tool can reduce the unacceptably long diagnostic delays for female patients.
In discussing sex-specific challenges, Dr Moltó reviewed how the diagnosis of axSpA can be more difficult in females, partly as a result of misleading symptoms suggestive of other conditions such as fibromyalgia, leading to diagnostic delays. Monitoring disease activity in females is also a challenge. These are relevant issues because females now comprise almost one-half of patients with axSpA and their burden of disease is at least comparable to that of males. Prof Rudwaleit outlined the modern concept of axSpA, which includes radiographic axSpA (r-axSpA) and nonradiographic axSpA (nr-axSpA), both of which have a similar burden of disease. Imaging, in particular MRI, is of great importance for diagnosis of nr-axSpA, and sacroiliitis on MRI determines progression. Nr-axSpA is not a self-limiting disease. Treatment options are growing and include treatment of extra-articular manifestations (EAM) such as acute anterior uveitis. Prof Landewé discussed evolving treatment targets and strategies for patients with axSpA, including the option to reduce the dose after remission is achieved.
![]()
Corrigendum: Raising the Bar for Optimal Management of Axial Spondyloarthritis
Chairperson: Robert Landewé
Speakers: Fabian Proft, Anna Moltó,Martin Rudwaleit
Acknowledgements: medical writing assistance was provided by Jennifer Taylor, London, UK
Original citation: EMJ Rheumatol. 2020;7[Suppl 2]:2-10.
Date correction published: 18.11.2020
In this article published in the 2020 Supplement 2 of EMJ Rheumatology, the following sentence is incorrect:
“Returning to the subject of disease manifestations, a meta-analysis published in 2019 showed no differences between r-axSpA and nr-axSpA in the prevalence of peripheral arthritis, dactylitis, enthesitis, and uveitis.32”
The sentence should read:
“Patients with r-axSpA and nr-axSpA share a similar clinical presentation except for peripheral involvement, which is more prevalent among nr-axSpA. However, this latter finding should be interpreted with caution, since peripheral manifestations might have allowed the classification of nr-axSpA in the absence of positive radiographic sacroiliitis, creating an artificial increased prevalence of such feature among this group.32”.
The error has been corrected in the PDF and HTML versions of the article. EMJ apologises for the error and any inconvenience caused.
![]()
This Catches the Eye – A Case Study
Doctor Fabian Proft
Dr Proft opened the symposium with the case of a 33-year-old female patient referred via the online self-referral tool ‘Bechterew Check’, an algorithm that calculates the probability of axSpA based on 15 questions.
The patient answered “yes” to both stem questions: chronic back pain lasting longer than 3 months and back pain starting before the age of 45 years. She also answered “yes” to four out of five inflammatory back pain characteristics: insidious onset, morning stiffness longer than 30 minutes, improvement with exercise and not with rest, and nightly awakening especially in the second half of the night.
The only question the patient did not answer with a “yes” to was alternating buttock pain. Out of eight questions on SpA parameters, the patient answered “yes” to only one question: “I suffer, or have suffered, from uveitis.” Taken together, she had both stem parameters, four out of five inflammatory back pain characteristics, plus the additional SpA characteristic of uveitis in the past. Therefore, the patient received a referral recommendation to a rheumatologist from the online self-referral tool.
First Appointment
At the first appointment, the patient reported 6 years of intermittent but chronic lower back pain. Despite multiple orthopaedic surgeon and primary care consultations, she had never been referred to a rheumatologist. Previously, a C-reactive-protein (CRP) test was negative and Bechterew’s disease was excluded. An X-ray at this time had shown some irregularities of the joint space on the left sacroiliac joint (SIJ). In February 2018, the patient had the first episode of an acute anterior uveitis of the right eye. Steroid eyedrops were prescribed by her ophthalmologist who also recommended a rheumatological check-up.
The symptoms of chronic back pain had worsened in the previous 8 weeks, and the patient had a pain score of 5–6/10 on a visual analogue scale (VAS). Ibuprofen was ineffective and caused gastrointestinal side effects. A quick CRP test, which gives a result in 2 minutes, showed an elevated level of 11.2 mg/L. Further diagnostics were ordered including CRP, erythrocyte sedimentation rate, human leukocyte antigen B27 (HLA-B27) genetic status, and MRI of the SIJ including short-TI inversion recovery sequences, and a T1 sequence. The patient was prescribed etoricoxibe 90 mg/day plus physiotherapy and a follow-up appointment was scheduled to take place after the MRI.
First Follow-Up
At the first follow-up, the patient still complained of intense lower back pain, with a VAS pain score of 6/10. Etoricoxibe was well tolerated but ineffective and physiotherapy had only just started. The diagnostics showed an elevated quick CRP value of 10.8 mg/L and positive HLA-B27 status. The MRI revealed active sacroiliitis of the SIJ with chronic changes on both sides, compatible with axSpA. The patient had a high Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and an Ankylosing Spondylitis Disease Activity Score (ASDAS) score of 3.4, categorised as high disease activity.1
A diagnosis of axSpA with high disease activity and failure to respond to nonsteroidal anti-inflammatory drugs (NSAID) indicated failure of Phase I treatment in the Assessment of SpondyloArthritis International Society (ASAS)–EULAR recommendations for management of axSpA;2 Phase II recommends initiating a biological disease-modifying antirheumatic drug.2 Treatment options for axSpA are TNF inhibition and IL-17A inhibition.2 For a young female with axSpA complicated by uveitis, the available evidence indicates starting with TNF inhibition therapy and, in particular, a monoclonal anti-TNF antibody.2
12-Week Follow-Up
Disease activity was re-evaluated after 12 weeks of treatment, as recommended by ASAS–EULAR.2 Shortly after the first injection, the patient experienced rapid improvement of lower back pain and morning stiffness, less fatigue, and could reduce the NSAID dosage. The injections were well tolerated with no side effects and there were no new uveitis episodes. The quick CRP value dropped to 4.2 mg/L, VAS improved to 1–2/10, BASDAI was 1.8, and ASDAS was 1.6. The 1.8 fall in the ASDAS score was a clinically important improvement.3 The patient was advised to continue TNF inhibition and reduce NSAID intake further if possible.
12-Month Follow-Up
One year after starting anti-TNF inhibition the patient reported no back pain, no stiffness, no new side effects, and no new uveitis episodes. The quick CRP value was very low, at 1.2 mg/L. All subjective assessments such as back pain (VAS: 0–1/10) and BASDAI (0.8) were also very low. This resulted in an ASDAS score of 0.9 and inactive disease.1
In summary, this case illustrated that the diagnostic delay in axSpA is still unacceptably long, and female sex is one of the predisposing factors for delay. New referral strategies are needed to improve early diagnosis and treatment initiation. A patient-based referral tool could be used in addition to existing physician-based referral strategies. In axSpA complicated with uveitis, monoclonal TNF antibodies are the treatment of choice. Calculating the ASDAS with the quick CRP value can be an important tool to implement treat-to-target in daily clinical routine.
Gender-specific Challenges and Approaches in the Management of Axial Spondyloarthritis
Doctor Anna Moltó
The challenges for females in axSpA management start with the diagnosis. The first reason is that ankylosing spondylitis (AS)/radiographic SpA has historically been considered a disease of young males. In addition, females tend to present with fewer structural lesions, particularly in the early stages of the disease.4 Symptoms can be misleading. Fibromyalgia causes widespread pain triggered at particularly tender points, which can be mistaken for points of enthesitis of axSpA and vice versa.5,6 This is a particular challenge in females because fibromyalgia is believed to be much more prevalent than in males. Objective signs, the hallmarks of the disease, can also be misleading in females. In a cohort of 35 healthy females, 77% displayed sacroiliac bone marrow oedema immediately postpartum and 60% fulfilled the ASAS definition of MRI sacroiliitis.7
When there is a diagnostic challenge it usually leads to greater diagnostic delay. Research in AS shows that the gap between age of disease onset and age at diagnosis is approximately 5 years in males compared with almost double that in females.8 Another study reported that 41% of females experienced a diagnostic delay of less than 2.3 years, while in 51% of females it was 2.3 years or longer.9
Disease monitoring tools raise further issues in females. Many studies have reported that females score higher on patient-reported outcomes (Table 1). At early stages of the disease, data from the DESIR study showed that females scored higher on all BASDAI questions compared to males, except on duration of morning stiffness, where there was no difference.10 They also scored higher on the Bath Ankylosing Spondylitis Patient Global Score (BAS-G) and Bath Ankylosing Spondylitis Functional Index (BASFI) scales, and their performance was worse on the short form-36 mental score, short form-36 physical score, Ankylosing Spondylitis Quality of Life Questionnaire (ASQoL), and Health Assessment Questionnaire for the spondyloarthropathies (HAQ-AS) score. The only comparable score between sexes was the CRP-based Ankylosing Spondylitis Disease Activity Score (ASDAS-CRP).10
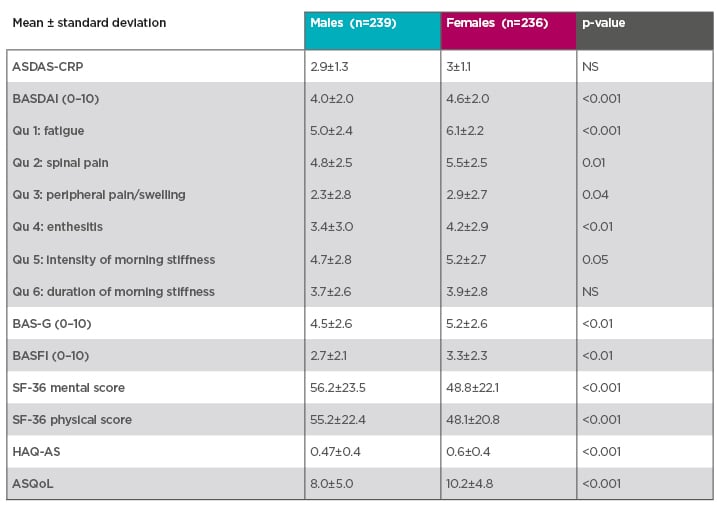
Table 1: Females tend to score higher on patient-reported outcomes.10
Prospective multicentre French cohort of patients with early inflammatory back pain suggestive of spondyloarthritis and fulfilling the Assessment of SpondyloArthritis international Society (ASAS) classification criteria for axial spondyloarthritis.10
Even in longstanding disease, females tend to present with higher patient-reported outcome scores, with the gap between males and females narrowing very late in the disease.4,11 In both early and longstanding forms, ASDAS is comparable between sexes, potentially because it includes CRP. However, ASDAS is correlated with MRI inflammation in males but not in females.12
Even more challenging is assessing disease activity when the patient has both axSpA and fibromyalgia. The PREDICT study demonstrated that patients with comorbid fibromyalgia were much less likely to respond to treatment with a TNF-α blocker.13 This is a particular issue for females, who have a higher prevalence of comorbid fibromyalgia compared with males.13
Addressing the challenges of axSpA in females is highly relevant, especially because data now show that the sex ratio of disease prevalence is approaching 1:1.4,14 Females have a worse burden of disease compared with males, even in the early stages, despite having less systemic inflammatory lesions. In the DESIR early axSpA cohort, females had higher scores on pain, tender joints, swollen joints, and enthesitis, but lower CRP levels than males.10 There were no differences between sexes in extra-articular involvement, response to NSAID, and HLA-B27 positivity.10
Female sex is prediction of a poor prognosis over time regarding the burden of the disease. Data from the DESIR cohort were used to determine trajectories of disease activity based on ASDAS over time.15 Five groups of patients were identified: 1) persistent very high disease activity; 2) persistent high disease activity; 3) changing from very high disease activity to inactive disease; 4) persistent inactive disease; and 5) persistent moderate disease activity.15 The proportion of females was much higher in the high disease activity categories. Being male was significantly associated with improvement to inactive disease, and persistently inactive disease.
The higher burden of disease in females has concrete consequences. Females have higher absenteeism, presenteeism, overall work impairment, and overall activity impairment compared with men.16 In the DESIR cohort, female sex was associated with almost twice the likelihood of an unfavourable functional outcome after 2 years.17 There is inconclusive evidence to explain why females have a higher burden of disease. Some argue that females have reduced access to biologics because of lower CRP values and less inflammatory lesions. When given biologics, females do respond, with corresponding lowering of the BASDAI score.16 In summary, diagnosing and monitoring disease activity in females with axSpA is a challenge. These issues are relevant because females now comprise almost one-half of patients with axSpA with a burden of disease at least comparable with that of males.
Continued Evolution of the Concept of axSpA
Professor Martin Rudwaleit
The modern concept of SpA is split into peripheral and axial disease; axSpA encompasses r-axSpA (formerly AS) and nr-axSpA.18 Nr-axSpA is defined by the absence of definite radiographic sacroiliitis on an X-ray.19 MRI helps make an early diagnosis because it can show active inflammation of the SIJ when an X-ray appears normal.19 However, every spot of bone marrow oedema on MRI does not reflect sacroiliitis. Small spots of bone marrow oedema have been described in athletes,20 cleaners,21 and soldiers,22 likely a result of mechanical stress. MRI investigations of the spine in healthy people in northern Germany found small bone marrow oedema lesions at the vertebral corners in 27.5% and fatty lesions in 81.4%.23 In axSpA, bone marrow oedema and fatty lesions are usually larger or more frequent.
The definition of sacroiliitis on MRI previously focussed on bone marrow oedema but data have since shown that the combination of bone marrow oedema and structural lesions is associated with higher confidence in the diagnosis.19,24,25 Sacroiliitis on MRI may be a disease-defining feature. Data from the ASAS and DESIR cohorts show that only patients with a positive MRI progress from nr-axSpA to r-axSpA, regardless of whether they have elevated CRP.26
Approximately 10–40% of patients progress from nr-axSpA to r-axSpA after a period of 2–10 years.27 Spinal progression occurs almost exclusively in patients with r-axSpA.28 Radiographic sacroiliitis is a requirement for developing syndesmophytes.28 Patients with syndesmophytes in the spine at baseline are more prone to progress further compared to those without.28 Regarding symptoms and disease manifestations, studies show a male predominance in r-axSpA compared with nr-axSpA.29 In addition, a higher proportion of patients with elevated CRP is consistently seen in r-axSpA compared with nr-axSpA.29 In contrast, there is no consistent difference between r-axSpA and nr-axSpA in subjective symptoms, i.e., pain, morning stiffness, fatigue, and quality of life,29 indicating the need to treat patients with nr-axSpA.
The landmark 52-week, placebo-controlled C-axSpAnd trial showed that certolizumab pegol was highly effective in treating active nr-axSpA.30 It also demonstrated that nr-axSpA is not a self-limiting disease.30 ASDAS major improvement at Week 52, the primary outcome, was achieved by 47% of patients treated with certolizumab pegol compared with 7% of patients treated with placebo.30 All patients received nonbiologic background medication.30 Patients with active disease who did not respond to treatment could escape to open-label treatment with certolizumab after Week 12; this occurred in 61% of the placebo arm compared to 13% of the certolizumab arm.30 A similar study design was used to investigate ixekizumab, an anti-IL-17 agent, in patients with active nr-axSpA.31 The ASAS40 response at Week 52 was significantly higher in patients taking ixekizumab compared with placebo.31 In this study, it is unclear why a similar proportion of patients chose the option of early escape in the placebo (59%) versus ixekizumab (around 40%) groups.31
Patients with r-axSpA and nr-axSpA share a similar clinical presentation except for peripheral involvement, which is more prevalent among nr-axSpA. However, this latter finding should be interpreted with caution, since peripheral manifestations might have allowed the classification of nr-axSpA in the absence of positive radiographic sacroiliitis, creating an artificial increased prevalence of such feature among this group.32 The prevalence of uveitis in SpA increases with disease duration; in longstanding SpA, more than 40% of patients have experienced at least one flare of uveitis.33 It has also been shown that uveitis is strongly associated with HLA-B27.33
Anterior uveitis in axSpA usually has an acute onset and affects one eye at a time.34 There is often spontaneous remission but recurrences do occur and may cause glaucoma, cataracts, or vision loss.35 Local eye drops (glucocorticoids) are very effective but in patients with more complicated causes of uveitis, or with many recurrences, there is limited efficacy of other treatments such as NSAID, sulfasalazine, or methotrexate.
A pooled analysis of data from placebo-controlled trials showed that TNF inhibition significantly reduced the frequency of acute anterior uveitis flares compared with placebo-treated patients.36 An open-label trial in patients with active AS demonstrated that the incidence of uveitis was reduced by 50% during treatment with adalimumab in all patients, with a similar degree of reduction in patients with a history of uveitis.37 The impact was even stronger in patients who had at least one uveitis flare in the last year.37
In the ongoing C-VIEW study, patients with uveitis and active axSpA received open-label treatment with certolizumab pegol.38 A prespecified interim analysis after 48 weeks of treatment found that the mean number of uveitis flares was reduced from 1.3 to 0.2 and the incidence of flares was reduced from 146.6 per 100 patient-years to 18.7 per 100 patient-years.38 This represents an 87% reduction in uveitis during treatment with certolizumab pegol.38
In summary, the modern concept of axSpA includes nr-axSpA and r-axSpA, which have a similar burden of disease. Imaging, in particular MRI, is of great importance for diagnosis of nr-axSpA and sacroiliitis on MRI determines progression. Nr-axSpA is not a self-limiting disease; treatment options are growing, and these include treatment of EAM such as anterior uveitis.
Evolving Treatment Targets and Strategies
Professor Robert Landewé
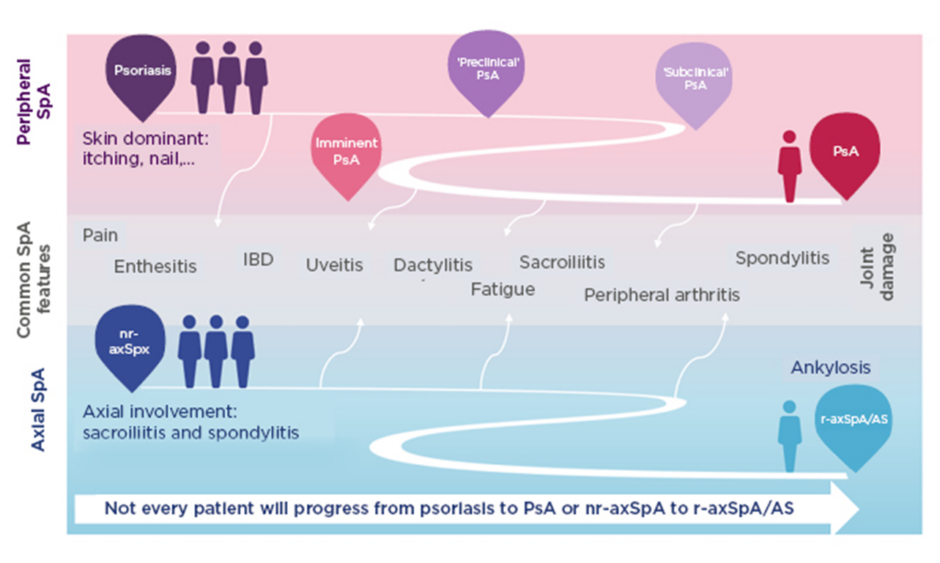
SpA encompasses heterogeneous inflammatory diseases with overlapping clinical features (Figure 1).39-43 Patients may develop predominantly axial or peripheral involvement; however, axial symptoms may also present in psoriatic arthritis and peripheral symptoms in axSpA.39-43 Pain is a hallmark of SpA but is often nonspecific. Chronic widespread pain affects 50% of patients and is more pronounced in females.44 Lower pain tolerance, also more common in females, is associated with worse disease activity, fatigue, and reduced spinal mobility.44 More specific indicators of axSpA are EAM including uveitis, psoriasis, inflammatory bowel disease, and nail disease,39,45,46 and peripheral articular manifestations such as peripheral arthritis, enthesitis, and dactylitis.39,45
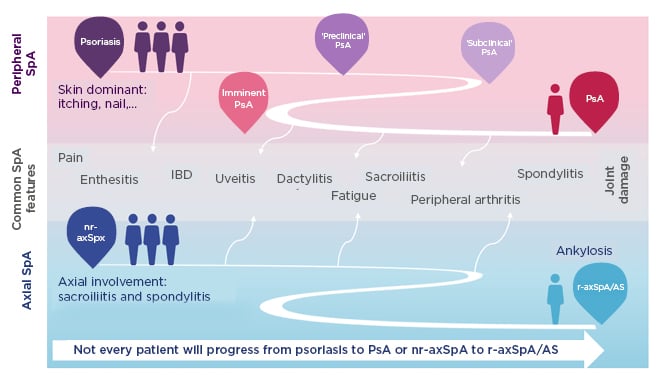
Figure 1: Spondyloarthritis encompasses heterogeneous inflammatory diseases with overlapping clinical features.39-43
Patients may develop predominantly axial or peripheral involvement; however, axial symptoms may also present in PsA and peripheral symptoms in axSpA.
AS: ankylosing spondylitis; IBD: inflammatory bowel disease; nr-axSpA: nonradiographic axial spondyloarthritis; PsA: psoriatic arthritis; r-axSpA: radiographic axial spondyloarthritis; SpA: spondyloarthritis.
Some signs and symptoms of SpA are directly a result of active inflammation, for example inflammatory back pain or raised CRP, but many symptoms are instead associated with chronic disease, such as chronic widespread pain, fatigue, general malaise, and depression. There is also accrual of radiographic damage in axSpA, i.e., syndesmophytes in the spine. Regardless of their origin, symptoms may lead to job loss, impaired physical function, anxiety, and depression.
Given the variety of symptoms and signs with inflammatory and noninflammatory causes, there is no single treatment for all patients with axSpA. Only some aspects of axSpA are influenced by drug treatment and an important question is: “should we start drugs as early as possible?” The potential role of early abrogation of inflammation in axSpA with reference to new bone formation has been amply discussed. The natural course of a fluctuating disease and continuous progressions of fatty lesions and new bone formation has been compared with hypothetical cases of late abrogation versus early abrogation.47 It has been hypothesised that early abrogation of inflammation leads to a lower likelihood of bone formation and thus a better outcome.47 However, there is no evidence to support the hypothesis and the “early is always better” axiom rests on strong beliefs but sparse data. It is important to be aware of overdiagnosis or misdiagnosis of axSpA. Escalating costs are another potential concern when starting earlier with more expensive drugs, and global inequity of access to drugs for patients should not be forgotten.
Regarding overdiagnosis of axSpA, an analysis in patients with early onset chronic back pain (SPACE) or inflammatory back pain (DESIR) demonstrated that up to 50% had SpA-like disease.48 In other words, they had symptoms of axSpA but few signs of chronic inflammation,48 meaning there was no indication for anti-inflammatory therapy. More than 50% of patients enrolled in DESIR had “SpA at risk” at most, and none of them developed true axSpA during a period of more than 5 years.48 It may be concluded that “SpA at risk” should not be treated with expensive biological disease-modifying antirheumatic drugs; these drugs cannot be expected to work because there is no sign of inflammation.
In a patient recently diagnosed with SpA, drug treatment should start with NSAID, which are widely available, cheap, provide symptom relief in more than 70% of patients with real axSpA, and are tolerated relatively well. It is also possible, but the evidence is inconclusive, that NSAID inhibit radiographic progression. Biologics are an asset for patients with axSpA with obvious inflammatory symptoms, despite treatment with NSAID. The current thought is that they likely have some positive effect on bone formation but, again, the evidence is inconclusive.
Another heavily disputed topic in axSpA is treat-to-target, which refers to choosing a target, usually remission or low disease activity, and intensifying treatment while the target has not been reached. An international expert consensus notes that this concept should be investigated further and recommends ASDAS as the clinical measure.49
Treat-to-target is also an axiom: it may work in axSpA but there is no proof. As with the “early is always better” axiom, the idea is based on strong beliefs but data to support the concept are sparse. There is the potential for overdiagnosis with the result that treatment is ineffective. Economic burden can result from medication switches and inequity in access to drugs remains an issue. Treat-to-target should only be pursued if there is certainty about the diagnosis of axSpA and only if there is inflammation that can potentially be suppressed. Treat-to-target is never a licence to start biologic drugs or JAK inhibitors.
In patients with axSpA who truly need TNF inhibitors, drug costs could be lowered by considering a reduced dose. In the C-OPTIMISE trial of patients with axSpA, remission was induced with the full dose of certolizumab pegol for a period of 48 weeks.50 Patients were then randomised to unchanged continuation of certolizumab pegol, complete withdrawal of certolizumab pegol, or a 50% dose reduction for a further 48 weeks.50 Patients in the placebo group relapsed.50 Patients on the full dose of certolizumab pegol continued to do well, and those on the half dose did equally well, with low rates of flares in both groups (4-weekly dose is not approved in the European Union [EU] ).50
In summary, most research in axSpA aims to treat inflammation with specific therapies but many patients have a high burden of symptoms with little inflammation. Some patients diagnosed with SpA actually have “SpA-like disease” and rheumatologists should be aware of two unproven beliefs, namely, “early is always better” and “treat-to-target.” Dose reduction may be an option after remission.