Meeting Summary
Metacognition is thinking about thinking, knowing about knowing, and being aware of your own awareness. It refers to the processes used to plan, monitor, and assess our own understanding and performance. By applying this metacognition concept and thinking critically about current beliefs and practices in the management of rheumatoid arthritis (RA), this symposium aimed to help rheumatologists think about how to positively impact patient care. Prof Andrea Rubbert-Roth introduced the meeting by looking at current approaches to the management and treatment of RA and the disconnect between the treatment goals of physicians and patients. Prof John Weinman provided an overview of the causes and extent of non-adherence, focussing on the role of patient beliefs and the use of consultations to facilitate better adherence. In the third presentation, Prof Daniel Aletaha applied the concept of ‘the ideal’ versus ‘the norm’ to three important areas in the management of RA: how we define remission, how we measure remission, and the minimally clinically important difference (MCID) in treatment outcomes as perceived by the patient. Prof Rubbert-Roth followed up with a review of the data on cycling or switching between different classes of biologic treatment and the use of patient characteristics and, eventually, biomarkers to guide the preference of clinicians for drugs targeting tumour necrosis factor (TNF) or other targets with overlapping but distinct signalling pathways, such as IL-6. Finally, Prof Weinman discussed the holistic care and treatment of patients with RA, emphasising the need for an empathic and collaborative approach to patient care.
Is it Possible to Achieve Better Disease Control in Rheumatoid Arthritis?
Professor Andrea Rubbert-Roth on behalf of Professor Leonard Calabrese
Since the 1990s, outcomes for patients with RA have steadily improved over time, with the treatment target for patients evolving from symptomatic relief, to prevention of damage and disability, and finally towards disease remission.1,2 These improvements in outcomes have occurred alongside advances in therapy and collaborative goal setting between rheumatologist and patient. Indeed, the rheumatology field may seem very fortunate, with a broad range of treatments and an established treat-to-target strategy that has transformed clinical remission from a long shot into an achievable target. In reality, only 30–40% of patients achieve clinical remission and for many patients their disease remains uncontrolled.3 In patients who respond inadequately to methotrexate and/or a first-line TNF inhibitor (TNFi), residual inflammation remains an important issue irrespective of subsequent treatment and represents a major unmet need (Figure 1).1
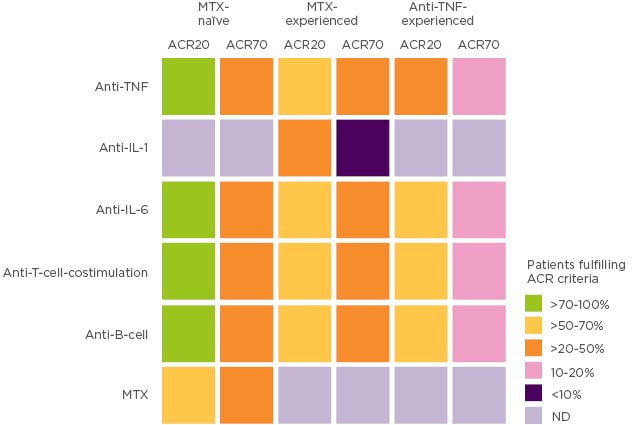
Figure 1: Patients fulfilling ACR criteria by prior RA treatment.
Adapted with permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nat Rev Rheumatol. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges, Smolen JS and Aletaha D, Copyright (2015).1 ACR: American College of Rheumatology; MTX: methotrexate; ND: not done; TNF: tumour necrosis factor.
The therapeutic options for RA will continue to expand, with cytokine and signal transduction targets remaining the predominant focus of drug development,4 but what else can rheumatologists do with what they already have? Could thinking differently about current thinking and practices in RA management offer another route to achieving better disease control, building upon past successes in managing this disease? Prof Rubbert-Roth believes a good starting point is to examine the disconnect between patient and physician perceptions of treatment goals and exploring ways to overcome this in clinical practice. Physicians have been taught that by achieving disease remission they can ultimately stop the development of radiographic progression, reduce physical disability, and have a positive impact on mortality.5-7 However, the most important aspects of care from the patient’s perspective are control of pain and fatigue and maintenance of physical function and health-related quality of life.6-10
During the course of a typical day, a patient with RA may experience a range of negative aspects of their disease that physicians may not necessarily be aware of, or may not directly address with the patient; for example, many patients with RA experience anxiety and depression, reduced sexual functioning, and may be limited in their work and social participation.6,11,12 The disconnect is clear when comparing the factors that drive patients’ and physicians’ global assessment scores. From the patient side, pain is the principal driving factor, whereas physicians may place more emphasis on number of swollen joints.13 This presents a scenario wherein the disease is well controlled in terms of inflammation, satisfying the physician, but the patient may still have residual pain and fatigue, and not be completely satisfied with their care. This amounts to an unmet medical need from the patient’s perspective.
In the context of treatment goals in RA, patients want their pain to be completely resolved, not just reduced, and to be able to do the activities they enjoy.14 Good QoL is paramount. Physicians, on the other hand, aim for no or very low levels of inflammation, no accrual of joint destruction, and no drug-related side effects, with QoL as a secondary concern.15 This suggests that good communication and greater collaboration between patients and physicians could positively impact the management of RA. Treating an inflammation-based target is undoubtedly important, but not good enough.15-18 A dual target strategy, a treat-to-target approach that takes into account of collaborative goal setting between physicians and patient, offers a way forward, in which the patient’s personal goals are also targeted through shared decision-making.19 For example, patient-perceived remission may include such goals as absence or reduction of symptoms, decreased daily impact of their condition, and a feeling of returning to normal.20 Prof Rubbert-Roth concluded by identifying gaps where changes could be made to positively impact the care of patients living with RA. The majority of these focussed on the patient physician interaction, emphasising the importance of collaboration, empathic communication, shared decision-making, identifying and managing patient concerns, considering their beliefs and adherence, and taking a whole-patient approach to their care. Through these collective actions, rheumatologists can provide a truly optimal, tailored treatment that meets therapeutic targets, including comorbidities and patient-reported outcomes (PRO).15
The Role of Patient Beliefs in Rheumatoid Arthritis Adherence and Therapy Optimisation
Professor John Weinman
Adherence has long been discussed as a public health issue but its impact is not routinely assessed in clinical practice and is often underestimated.21,22 A 2018 working paper published by the Organisation for Economic Co-operation and Development (OECD) concluded that poor adherence is “a major public health problem”, contributing to 200,000 early deaths annually in Europe, with an estimated cost of €125 billion.22 Reasons for this include a lack of awareness among practicing physicians and unwillingness to take ownership of the problem.22,23
Adherence can be separated into three distinct phases: uptake, implementation (how patients integrate the treatment into their lives), and persistence (how long patients stay on treatment).24 In patients with chronic metabolic diseases, up to 31% never start their prescribed treatment (‘primary non-adherence’). Of those who do start treatment, only 50–70% are regularly adherent and less than half persist on treatment for 2 years.22 Reported rates of non-adherence vary considerably in RA, partly due to differences in study methodology.25 In a systematic review of 52 studies, as many as two-thirds of patients stopped biologic treatment within 1 year, with overall adherence of 41–81%.25 Evidence shows that the impact of non-adherence in RA manifests not only in increased costs of healthcare but also in a clear effect on remission, likely falling short of patients’ personal goals.26,27 Non-adherent patients are only half as likely to achieve remission, and take twice as long, as patients who take treatment as directed.27
To determine the reasons for non-adherence, it is necessary to look not only at the drivers of patient behaviour, but also at the contribution of physicians and healthcare systems. A convenient way to look at drivers of patient behaviour is to apply a Capability, Opportunity and Motivation (COM-B) model.28,29 Each of the three components are important in driving patient behaviour in RA, but perhaps the most influential are the patient’s own perceptions. These can be categorised as perceptions of illness (i.e., patients’ beliefs about the nature, cause, consequences, timeline and cure/control of their condition); perceptions of treatment (i.e., do they doubt its necessity and/or do they have concerns about potential adverse effects?); and beliefs about self-efficacy (i.e., are they confident in their ability to continue taking the treatment over time?).28 Studies of patients with chronic diseases, including RA, clearly show that patients who doubt the necessity of day-to-day treatment and concerns about safety are least likely to adhere to treatment.29-32 Taken together, the evidence shows that not only can beliefs vary enormously between patients, but they can also vary within the same patient over time as the pattern of treatment and treatment response changes. Prof Weinman emphasised the importance of not adopting a ‘one size fits all’ approach to interventions to improve adherence, but instead working to identify the issues that apply to each individual patient and then using personalised behaviour change interventions.33
From the physician’s perspective, an initial default thinking when a patient is not responding to a certain treatment is to increase the dose or switch to an alternative. It is unusual for physicians to check whether the patient has actually been taking their treatment as directed.22 Even when the question is asked, it is often asked in a way that patients feel obliged to give a misleading answer. Research also shows that adherence is not easily intuited; in one study, the physician’s beliefs about which of their patients are non-adherent were no more accurate than chance.34 There is a clear need for tools and training to improve open discussion between physicians and patients on individual adherence issues and how to manage these collaboratively. Physicians should periodically check patients’ understanding of treatment, using patient-friendly language, and take steps to improve this if needed. Steps may include providing a clear rationale for the necessity of the treatment, eliciting and responding to patient concerns, agreeing on a practical plan for how, where and when to take treatment, and identifying potential barriers.31
The Ideal versus The Norm: What Does Minimally Important Difference Mean and Why is This Important in Management of Rheumatoid Arthritis Today?
Professor Daniel Aletaha
Prof Aletaha applied the concept of ‘the ideal’ versus ‘the norm’ to three important areas in the management of RA. In the context of clinical remission, the question today is not necessarily whether remission is too ambitious but whether it is not ambitious enough. Could subclinical remission, also known as imaging remission, become the new ideal?15 Randomised studies exploring whether structural and functional outcomes are significantly superior in patients who go beyond clinical remission to achieve subclinical remission have so far yielded negative results.35-37 In the ARCTIC study, ultrasound tight control (defined as clinical remission and no ultrasound power Doppler signal) was not significantly superior to conventional tight control for the composite primary endpoint of disease activity score (DAS) <1.6, no swollen joints and non-progression of radiographic joint damage at 16 and 24 months.35 Similarly, the TaSER trial in newly diagnosed patients with RA or undifferentiated arthritis randomised to clinical remission or imaging remission (defined as total power Doppler joint count ≤1) found no significant difference between the two groups for any clinical outcome.36
In the third study, IMAGINE-RA, imaging remission (defined as no evidence of bone marrow oedema on magnetic resonance imaging [MRI]) also failed to demonstrate superiority over conventional tight control on the primary endpoints of remission and radiographic non-progression. However, statistically significant differences were reported for four of the secondary outcomes (American College of Rheumatology-European League Against Rheumatism [ACR-EULAR] Boolean remission, swollen joint count, patient global visual analogue scale assessment, and change in Health Assessment Questionnaire [HAQ] score) after 2 years of treatment.37 Overall, while acknowledging that treating to an imaging remission target may be of benefit in some patients, the current evidence does not support the use of more intensive monitoring and therapy in addition to conventional tight control. On a practical level, repeat MRI scans may not be feasible in clinical practice.
Prof Aletaha also explored ‘the ideal’ versus ‘the norm’ in terms of how we measure remission. Clinical studies commonly measure remission using DAS in 28 joints (DAS28) based on C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR), but neither outcome is recommended by ACR-EULAR due to lack of specificity.38 Even with adjusted cutpoints, around half of patients in remission according to DAS28-ESR (≤2.2) and around 30% of patients in remission according to DAS28-CRP (≤1.9) had at least one swollen joint, compared with only 10% of patients when remission was based on the ACR-EULAR recommended Clinical Disease Activity Index (CDAI).39 The problem with DAS28 remission is not the cutpoints but the strong weighting given to the acute phase response. This is clearly shown when comparing DAS28 remission responses with cytokine-based versus non-cytokine-based biologics. In the ATTAIN and REFLEX trials of the non-cytokine-based biologics abatacept and rituximab, respectively, DAS28 remission rates were similar to AC70 response rates, whereas in the RADIATE trial of the IL-6 receptor inhibitor tocilizumab, more patients achieved DAS28 remission than achieved an ACR50 response.40-42 Thus, DAS28 remission rates depend not only on efficacy but also on type of intervention.
The Boolean criteria recommended by ACR-EULAR are not infallible either. An estimated 61% of patients who are ‘near-misses’ for clinical remission (that is, patients who fulfil only three of the four ACR-EULAR Boolean criteria) fail to reach remission because of high patient global assessment (PtGA) scores. Pain is highly predictive of near-misses related to PtGA, and this is true regardless of whether the pain is related to inflammation or not.43 Given that there is a clear link between non-inflammatory pain and depression – the leading comorbidity in patients with RA44 – it is useful to first assess the impact of pain and depression before deciding on a treatment change in patients repeatedly failing objective-established remission criteria.
Finally, Prof Aletaha discussed the concept of the MCID in RA. MCID is typically defined as the smallest difference in a domain score of interest that patients perceive to be beneficial (in the absence of troublesome side effects and excessive cost) that would mandate a change in management.45,46 It is important to understand that the patient is the anchor of this definition, not least because it necessarily applies that MCID is dependent on baseline disease activity. This dependence has been demonstrated clearly by registry analyses. For example, data from a Norwegian registry identified the MCID cutpoints for improvement of CDAI as 1.8 for low disease activity, 7.3 for medium disease activity, and 17.8 for high disease activity.47 By comparison, an analysis of the Canadian Early Arthritis Cohort identified the same MCID cutpoints as 1, 6, and 12, respectively.48
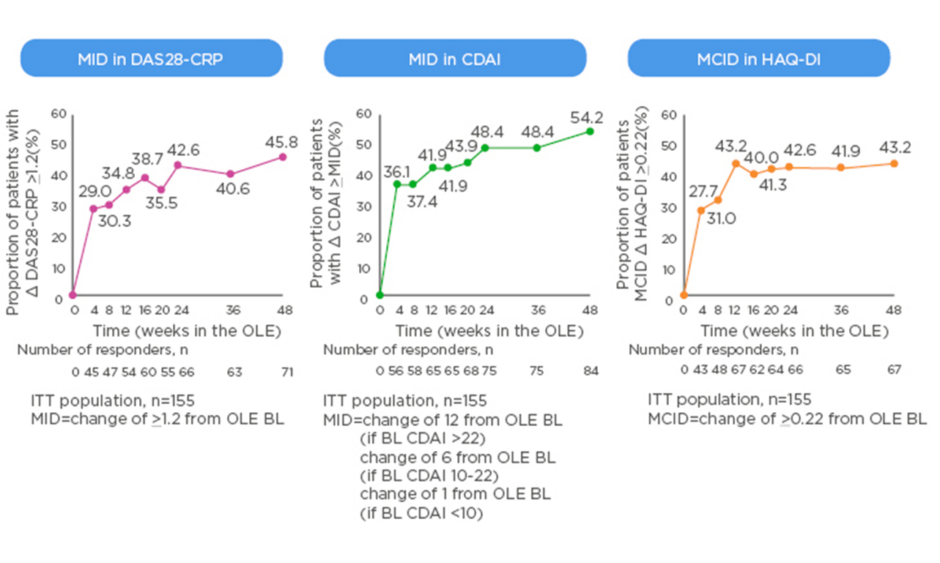
The MCID is valuable in the context of clinical trials as it offers a useful way to track disease activity from the perspective of the patients, for example when switching to a new biologic. New data from the open-label extension of the MONARCH trial show that patients switched from adalimumab to sarilumab experienced clinically meaningful improvements in DAS28-CRP, CDAI, and HAQ-DI that generally increased over time (Figure 2).49 Rheumatologists know from experience that the 3-month timepoint is important; the question is how much change at 3 months do physicians ideally want to see to reassure them about continuing the same therapy or regimen rather than switch to another. A pooled analysis of patient-level data from clinical trials found that achieving a minor response (e.g., ACR20 or a 50% improvement in Simplified Disease Activity Index [SDAI 50%]) at 3 months is associated with very low negative likelihood ratios for achieving SDAI low disease activity or remission at 6 months.50 On the other hand, rheumatologists can be confident in continuing the same therapy if the patient has a major response (e.g., ACR70, SDAI 85%, or EULAR good response) at 3 months.
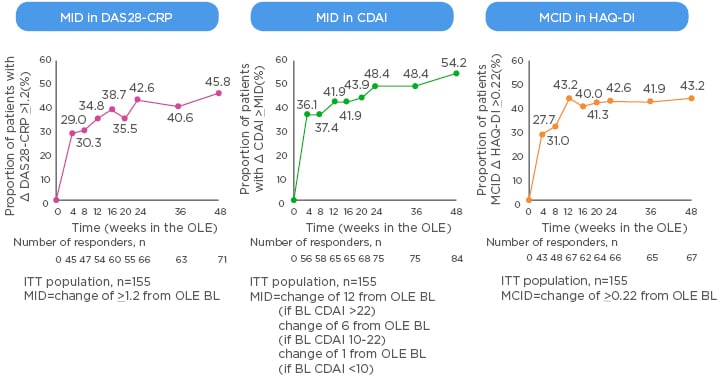
Figure 2: Minimally important difference in switching group from start of open-label extension of the MONARCH study.
BL: baseline; CDAI: Clinical Disease Activity Index; CRP: C-reactive protein; DAS: Disease Activity Score; HAQ-DI: Health Assessment Questionnaire – Disability Index; ITT: intent-to-treat; MID: minimally important difference; OLE: open-label extension.
eproduced with permission from Burmester et al.49
Changes in Daily Rheumatoid Arthritis Practice: Dealing with Loss versus Gain in Switching versus Cycling
Professor Andrea Rubbert-Roth
TNFi remain the most commonly used biologics in the management of patients with RA, supported by extensive experience and a wealth of data on long-term safety, cardiovascular benefits, and broad efficacy for spondyloarthropathies and other inflammatory joint diseases. Following the failure of a TNFi, EULAR recommends that patients be switched to an alternative drug class with a different mode of action (MOA) or cycled between drugs within the same class to try to mitigate against loss of efficacy.51 In the absence of biomarkers to truly personalise treatment, rheumatologists are faced with the challenging task of selecting an appropriate strategy that considers all of the clinical factors as well as the patient factors. From the patient perspective, drug selection may be driven by comorbidities and/or need for monotherapy, as well as the patient’s beliefs and overall goals. The availability of multiple TNFi and, recently, TNFi biosimilars may also influence prescribing habits.
Cycling to a second TNFi can be efficacious, as demonstrated in the EXXELERATE trial in which primary non-responders to certolizumab pegol were switched to adalimumab, and vice versa, with no washout period.52 Although the trial was negative, in that it failed to demonstrate superiority of certolizumab pegol to adalimumab, it had important implications for TNFi cycling in clinical practice. It is expected that around 30% of patients receiving their first TNFi will fail to achieve an ACR20 response.53 In EXXELERATE, a further drop-off in patients responding to treatment was demonstrated in patients who cycled to a second TNFi, with ACR20 response rates of 40–44%.52
There is now a wealth of data suggesting that switching to a different MOA may improve clinical outcomes and PRO. EULAR currently recommends switching drug class in patients who experience failure of two successive TNFi,51 but should clinicians be switching sooner? The Rotation or Change trial was designed to answer this question in a head-to-head study in patients randomised to cycling to another TNFi or switching to a non-TNFi biologic. The primary endpoint of EULAR good or moderate response at Week 24 was met by 69% of patients who switched and 52% of patients who cycled (p=0.004). At Week 52, switching was statistically significantly more effective than cycling across all secondary efficacy endpoints (EULAR good or moderate response, DAS28-ESR remission, and DAS28-ESR low disease activity).54
The benefits of switching rather than cycling are supported by results from placebo-controlled trials of non-TNFi biologics, conducted in patients who had an inadequate response or were intolerant to prior TNFi (TNF-IR). In the RA-BEACON study, the JAK1/2 inhibitor baricitinib provided rapid and sustained clinical benefit in TNF-IR patients, with an ACR20 response rate of 46% at Week 24.55 In comparison, TNF-IR patients treated with the IL-6 inhibitor sarilumab in the TARGET study had an ACR20 response rate of 61% at 24 weeks.56 Switching to an alternative MOA is also supported by registry data57,58 and long-term drug retention rates.54,59 For example, in the Canadian Rhumadata registry, switching to tocilizumab had a 4-year retention rate of 44.3% compared with rates of 27.2–37.1% when cycling through TNFi.59
Returning to her earlier point about biomarkers, Prof Rubbert-Roth introduced new data from the MONARCH study indicating that IL-6 may be a potential biomarker for guiding clinical decision-making in patients with RA. High baseline levels of IL-6 were associated with greater improvements in PRO for sarilumab versus adalimumab.60 Taken together with previously reported evidence of a predictive relationship between baseline levels of IL-6 and greater response to sarilumab,61 these results suggest an emerging patient profile for responders to treatment. In addition, a post-hoc analysis of data from TARGET and MONARCH shows a more pronounced reduction in glycosylated haemoglobin (HbA1c) with sarilumab versus placebo or adalimumab (Figure 3), irrespective of diabetic status. Notably, HbA1c reductions were greatest with sarilumab monotherapy in patients with high baseline levels of IL-6.62 Patients on biologic monotherapy are an important group to consider as methotrexate is frequently stopped because of side effects. 63 Clinical trial evidence favours the use of an IL-6 receptor or JAK inhibitor in these patients.64,65
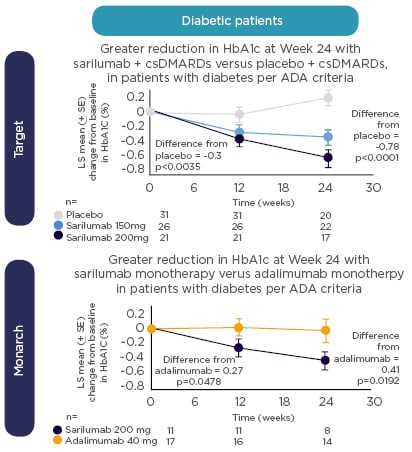
Figure 3: Change from baseline in HbA1c in patients treated with sarilumab versus placebo in the TARGET study and sarilumab versus adalimumab in the MONARCH study.
HbA1c was systematically collected at baseline and Weeks 12 and 24. ADA diagnostic criteria: fasting glucose ≥7 mmol/L or baseline HbA1c ≥6.5% ADA: American Diabetes Association; csDMARD: conventional synthetic disease-modifying antirheumatic drug; HbA1c: glycated haemoglobin.
Reproduced with permission from Genovese M et al.62
Holistic Care of Patients with Rheumatoid Arthritis
Professor John Weinman on behalf of Professor Leonard Calabrese
Prof Weinman argued that empathy should be taken seriously as a core skill that has real value in disease management. Research shows that physicians often miss opportunities to respond empathetically to their patients, leaving them unsatisfied.66 Qualitative research has shown that patients with RA who feel that no one is listening to them often come away from consultations feeling negatively, not only about their care but also about their ability to self-manage their condition.67
The concept of empathy in the context of patient care can be broken down into three core components: developing an understanding of the patient’s experiences, concerns, and perspective; having the capacity to communicate this understanding; and showing an intention to help.68 Recommendations for physicians include being mindful of eye contact, facial expression, posture, affect and tone of voice when speaking to patients, making sure to hear the whole-person perspective, and responding in a way that lets them know they have been heard.69
Interventions to increase empathic communication have been successful.66 Research shows that physicians who incorporate core empathy skills in routine practice feel more personal growth and greater job satisfaction as a result, and are less likely to burn out.68,70 Patients also benefit, not only in terms of reduced anxiety and greater satisfaction with care – a crucial component of adherence – but also in better clinical outcomes.71-73 In addition, evidence suggests that the perception of empathic communication as imposing an extra time burden on physicians is false. One study found that use of one empathic statement during outpatient visits can save 1.5–2 minutes per consultation, depending on the medical or surgical nature of the visit.74
Further research into empathy in the context of rheumatology is needed to determine the clinical correlates of empathy and the role of empathetic communication in the management of RA. Ideally, the need for empathy skills should be addressed early on during rheumatology training.
Conclusions
Professor Andrea Rubbert-Roth
Despite advances in the treatment of RA only 30–40% of patients achieve remission and for many patients their disease remains uncontrolled.3 The impact of non-adherence on disease control and patients’ personal goals is often underestimated. Non-adherent patients are only half as likely to achieve remission, and take twice as long, as patients who take treatment as directed.27 Rheumatologists need to be aware of the causes and extent of non-adherence, and to make use of consultations to facilitate informed adherence.25,28 When considering the patient experience, it is important to note that the MCID is, by definition, the patient perception of improvement and depends on baseline level of disease activity.45,46 The MCID is therefore a useful way to track disease activity from the patient perspective, for example when switching to a new biologic. In patients with an inadequate response to first TNFi – who may have non-TNF-driven or TNFi-resistant disease – switching to a different MOA may improve outcomes.53,54 A lack of biomarkers leaves rheumatologists with the challenging task of personalising treatment based on a broad range of factors. Patient characteristics (e.g., comorbidities) may guide physicians’ preference for non-TNF-targeted drugs.60,62 Emerging data suggest that baseline IL-6 levels may have utility as a biomarker for treatment response.60-62 Clinical trial evidence favours the use of an IL-6 receptor or JAK inhibitor in patients on biologic monotherapy.64,65 In conclusion, by thinking critically about current beliefs and practices in management of RA and collaborating with patients in an empathetic way to identify and address suboptimal disease control, it is possible to do more to positively impact patient care.