Author: Eleanor Roberts
Beeline Science Communications, Ltd, London, UK.
Disclosure: Roberts has received remuneration from EMJ for writing.
Support: This article was funded by an educational grant from Pfizer.
Citation: EMJ Rheumatol. 2021;8[Suppl 5]:2-10.
Summary
The chronic inflammatory diseases axial spondyloarthritis (AxS) and psoriatic arthritis (PsA) involve symptoms such as axial skeleton pain alongside enthesitis and arthritis. At the European Alliance of Associations for Rheumatology (EULAR) 2021 Virtual Congress (2nd–5th June), a number of abstracts were dedicated to AxS and PsA. A few of the presentations highlighted how diagnosis and evaluation of these conditions was potentially hampered by diagnostic delay and problems distinguishing specific AxS or PsA symptoms. It was discussed over some of these presentations that factors that should be taken into account when diagnosing these conditions include not only symptoms but also age, sex, and comorbid conditions. Treatment for AxS and PsA includes more traditional non-steroidal anti-inflammatory drugs (NSAID) and disease-modifying antirheumatic drugs (DMARD), alongside relatively newer medications such as TNF inhibitors (i), ILi, and JAKi. Several of the studies discussed at the Congress presented details of both Phase II and III and real-world studies of several of these drugs and detailed how PsA and AxS symptoms can be alleviated by such in both the short- and long-term. Overall, at the EULAR 2021, unmet needs highlighted included better understand regarding the pathophysiology of AxS and PsA; earlier recognition and diagnosis; a treat-to-target (T2T) approach; and a holistic view of disease burden.
Introduction
The chronic inflammatory disease AxS primarily affects the axial skeleton and typically presents with lower back pain, enthesitis, and arthritis.1 In some patients, structural damage can be shown radiographically (radiographic AxS or ankylosing spondylitis); however, such damage is not shown (non-radiographic AxS) for others.1 PsA, another type of spondyloarthritis, is particularly associated with reactive arthritis and inflammatory bowel disease (IBD).1 Presentation may include musculoskeletal pain and inflammation in the axial skeleton, digits, and entheses, as well as skin and nail disease.2
At the EULAR 2021 Virtual Congress (2nd–5th June), a number of abstracts were dedicated to AxS and PsA. Here, a few of the studies presented involving the landscape of healthcare, disease identification, diagnosis, management, and unmet needs will be discussed.
Diagnosis and Evaluation
Psoriatic Arthritis
Investigation of PsA may include the use of screening instruments.3-5 Gercek Can et al. carried out a systematic literature review of several of these and found wide variability in sensitivity, specificity, and positive/negative predictive values, both between instruments and between different studies with the same instrument. Can et al. concluded that “there is an unmet need for a screening instrument with a better performance in all disease domains.”6
Fazira Kasiem et al. presented a dermatology-specific, health-related quality of life questionnaire that could be used to identify high psoriasis burden in PsA.7 A positive answer and rating to the statement “I am embarrassed by my skin condition” led to the statement “I tend to stay at home because of my skin condition,” with a positive response here leading to “Over the past week, has your skin prevented you from working or studying?” The second topline question was: “Over the last week, how itchy, sore, painful, or stinging has your skin been?” A positive response led to patients being asked to rate if or how much their skin was irritated. Kasiem et al. concluded that, with just these 2–5 questions, “both psychosocial burden of psoriasis and burden of physical symptoms can be easily identified.”7
Around 21% of people with psoriasis develop PsA8 and two studies examined how to best differentiate and characterise this condition. In an analysis of real-world data from the British Association of Dermatologists Biologic and Immunomodulators Register (BADBIR),9 William Tillett examined characteristics of patients with psoriasis, with (n=3,287) or without (n=5,634) PsA. They found prevalence of several comorbidities (including depression, obesity, diabetes, and hypertension) in those with PsA and a reduced ability to work. They also found a higher likelihood of receiving ustekinumab or a DMARD and concluded that the results “potentially indicate a higher inflammatory and quality of life burden in patients with psoriasis and PsA.”10 Diego Benavent et al. analysed data from patients with PsA (n=367) and patients with AxS (n=2,651) involved in the ASAS-perSpA study, which includes 68 centres worldwide, and found that diagnosis of axial PsA, as opposed to AxS, was significantly associated with older age, peripheral arthritis, and dactylitis, with a negative association with uveitis, IBD, and human leukocyte antigen *B27 (HLA*B27) status. Axial involvement in PsA was associated with absence of psoriasis, males, and elevated C-reactive protein (CRP).11
Enthesitis is a typical feature of PsA2 and Maria-Antonietta D’Agostino et al. discussed the use of an enthesitis score using power Doppler ultrasound (PDUS), developed by the Outcome Measures in Rheumatology (OMERACT) US group.12 Utilising data from the ULTIMATE trial that compared the ILi secukinumab to a placebo,13 they found that up to 88% of the cohort had enthesitis detected by PDUS at baseline. Changes from baseline to Week 12 were found when using the Global OMERACT-US Enthesitis Score, which encompasses PDUS findings, and it was proposed that this score could potentially be used in clinical trials.14
Axial Spondyloarthritis
AxS diagnosis is a combination of positive and negative test results to rule in AxS as a potential cause of symptoms and rule out other aetiologies.15 In patients where AxS was suspected by a rheumatologist, a study presented by Denis Poddubnyy et al. was carried out to evaluate the diagnostic value of AxS parameters and their combination using data from the Optimal Referral Strategy for Early Diagnosis of AxS cohort (n=361). Here, an online spondyloarthritis probability calculator was utilised to generate a likely probability percentage based on factors such as presence or absence of inflammatory back pain, good response to a NSAID, HLA*B27 positivity, CRP elevation, and structural changes and/or active inflammation on MRI. They found that the results of these diagnostic tests interacted with each other to affect the probability of an AxS diagnosis.16
Another area of interest in AxS diagnosis is the association with IBD.1 Jonas Sagard et al. investigated gut dysbiosis via faecal sampling and found this in 33% of patients with AxS compared with 17% of controls (p=0.027). As gut dysbiosis, and higher levels of such, was associated with worse disease activity and outcome scores, Sagard et al. concluded that for those with AxS, “gut dysbiosis may be a novel biomarker for severe disease.”17
Diagnostic Delay
Diagnostic delay is prevalent in people with AxS18 and several presentations dealt with this issue. Marco Garrido-Cumbrera et al. analysed data from 2,846 patients included in European Map of Axial Spondyloarthritis (EMAS), a 2017–2018 online survey across 13 European countries. They found diagnostic delay to be, on average, 7.4 years. They also found that patients visited a mean of 2.6 healthcare professionals before diagnosis such as general practitioners (GP), rheumatologists, physiotherapists, orthopaedic surgeons, or osteopaths, and many had multiple diagnostic tests including MRI, ultrasound, and/or CT scans, X-rays, and HLA*B27 status investigation. Final diagnosis was most commonly made by a rheumatologist (78.4%) but in 14.9% of patients, AxS was diagnosed by their GP.19
Once AxS is identified, patients may be referred to a rheumatologist.1 However, in an investigation of patients in England and Wales, Mark Russell et al. used National Early Inflammatory Arthritis Audit (NEIAA) data to show that median time to rheumatology assessment after referral was greater in those with AxS (36 days) compared with those with rheumatoid arthritis (24 days; p<0.001). They concluded that “diagnostic delay remains a major challenge in AxS” and highlighted an unmet need for patient education and specialist clinics.20
A literature review by Dale Webb et al. identified four potential reasons for diagnostic delays: primary care practitioners not recognising AxS features; patients not knowing that their chronic back pain may be AxS; referrals to non-rheumatologists who do not promptly recognise AxS; and rheumatology and radiology teams not optimally requesting or interpreting investigations. Webb suggested that a ‘Gold Standard’ time to AxS diagnosis would be 1 year.21
Diagnostic delay may impact both the patient directly and healthcare cost. Eduardo Flores-Fernández et al. carried out an observational, retrospective analysis of patients with AxS who were referred to their Spain-based rheumatology department and found that for patients who were initially referred to another specialist, costs were double that of those initially referred to rheumatology. This, Flores-Fernández et al. stated, demonstrated an “unmet need of improving the management and referral…from the GP to the rheumatologist.”22 One potential way to avoid diagnostic delay and improve the patient journey for those with psoriasis and PsA is, according to a study by Kalliopi Klavdianou et al., to assess patients in a combined Dermatology–Rheumatology clinic, as they have done in Greece. By doing this, they found patients received an earlier PsA diagnosis and more efficacious treatment.23
Treatment
Treatment for AxS and PsA includes more traditional NSAIDs and DMARDs, alongside relatively newer medications that target the inflammatory causes of these conditions such as TNFi, including infliximab, adalimumab, and certolizumab; ILi, including secukinumab and ixekizumab; and the JAKi upadacitinib.1,2,24 A number of EULAR 2021 presentations discussed approaches to treatment as well as studies examining specific medications.
In a study by Alexis Ogdie et al., the burden and importance of common PsA symptoms and disease impacts was investigated, alongside what patients’ treatment preferences were. They found that the most bothersome symptoms were joint pain, lower back/spine pain, tender/painful tendons/ligaments, and fatigue/tiredness. The most important disease impacts patients wanted improving with treatment were the ability to perform physical and daily activities and to live and function independently; sleep quality; the unpredictability of disease flare-ups; and emotional well-being. Ogdie et al. concluded by saying that “these findings can serve to better inform development of new therapies and guide shared patient-provider treatment decision making.”25
A treat-to-target (T2T) strategy has been recommended for AxS treatment26 and L Xu et al. examined the awareness and implementation of such guidelines in China using a web-based questionnaire. They found significant disparities in disease assessment periods and criteria, with only 62.4% of respondents stating they were familiar with a T2T strategy. While many rheumatologists applied this strategy, of those who did not, it was disclosed that they did not fully understand T2T, highlighting an unmet need for education on this strategy.27
Overall treatment recommendations for PsA, based upon best scientific evidence and including diverse stake holders, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) were presented by Coates et al. Recommendations were separated by PsA symptoms, with individual medication recommendations for each (Figure 1).28 One key factor updated from the 2009 recommendations29 was that a greater range of comorbidities was included as needing investigation, as well as considering how they impacted PsA.28
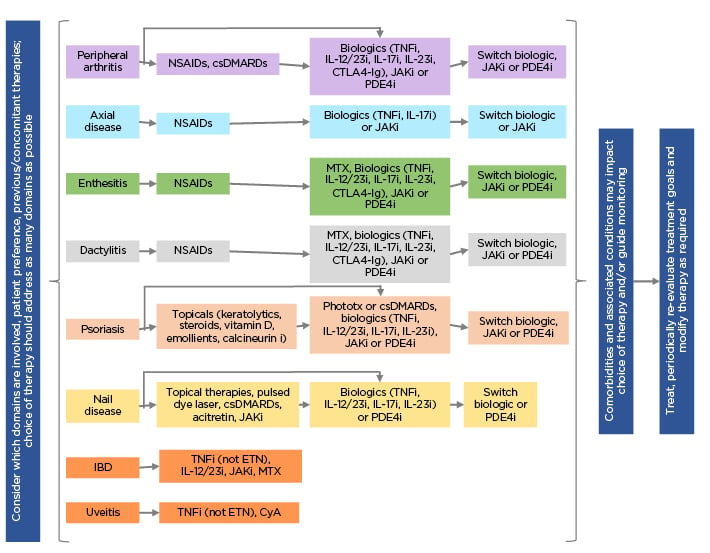
Figure 1: Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) recommendations 2021.28
csDMARDs: traditional disease-modifying antirheumatic drugs; CTLA4-Ig: cytotoxic T-lymphocyte antigen 4-immunoglobulin; CyA: cyclosporin A; ETN: etanercept; i: inhibitor; IBD: inflammatory bowel disease; MTX: methotrexate; NSAID: non-steroidal anti-inflammatory drug; PDE4i: phosphodiesterase 4 inhibitor; Phototx: ultraviolet B phototherapy.
TNF Inhibitors
TNFi are standard treatments for AxS1 and PsA and a few of the presentations at EULAR 2021 investigated these either as a class or individually.
Poddubnyy et al. evaluated patients from the German Spondyloarthritis Inception Cohort (GESPIC), with radiographic and non-radiographic AxS treated with a TNFi. They found that while TNFi treatment was significantly associated with a positive change in radiographic spinal progression scores compared with patients who had not received a TNFi, the effect was ‘time-shifted’, such that this was shown only after at least a 2-year time period. They postulated that this could be related to “the immediate and long-lasting process of bone repair that follows resolution of inflammation and preceded development of new bone” and suggested that at least 4 years of observation may be needed to see new bond formation following TNFi treatment.30 Also using GESPIC data, Murat Torgutalp et al. found that after 2–4 years, TNFi use was also associated with retardation of sacroiliitis progression in AxS patients.31
Cédric Lukas et al. investigated whether the use of TNFi could be tapered in patients with low disease activity for at least 6 months and normal CRP levels and found that tapering may be feasible, with a similar rate of disease flaring as those without tapering.32 Similarly, Lianne Gensler et al. examined data from the C-OPTIMISE study of patients with AxS treated with 200 mg certolizumab pegol every 2 weeks. In patients who achieved sustained remission during the 48-week study (n=313), who continued into a further 48 weeks treatment, 85.6% on the same dose regimen did not experience a flare compared with 80.0% receiving 200 mg certolizumab pegol every 4 weeks and 23.1% receiving a placebo. Disease activity was similar with either dosing regimen of certolizumab pegol over this time period, showing the potential use of a reduced maintenance dose.33
Another study, carried out by Ekaterina Agafonova and Shandor Erdes, specifically examined how the symptom of coxitis in AxS may be affected by combined TNFi plus NSAID therapy. They found that of 19/27 patients in the combined therapy group who had coxitis at baseline still showed this after 2 years of treatment. For those in the NSAID-only group, respective numbers were 28 at baseline and 24 after 2 years. While a higher percentage in the combined group no longer showed coxitis after 2 years, as combination therapy did not improve coxitis in many, Agafonova and Erdes concluded that “further studies are needed to improve the effectiveness of treatment of coxitis in AxS.”34
Another interesting study that looked at one particular factor in AxS, gut dysbiosis, was presented by Maria Ditto et al. In 20 patients with AxS, they found that TNFi could potentially reverse gut dysbiosis as evidenced by 6 months of such therapy leading to significant increases in the levels of bacteria of the Lachnospiraceae family and the Coprococcus genus and a decrease in Proteobacteria and Gammaproteobacteria.35
Louis Bessette et al. looked more specifically at the impact of the TNFi adalimumab (n=452) on disease activity and patient-reported outcomes versus non-biologic therapy (DMARD or NSAID; n=187) in the COMPLETE-AS trial, a Canadian observational study in patients with radiographic AxS. While most baseline demographics were similar, patients prescribed adalimumab had significantly higher baseline disease severity. The adalimumab group had a 60% reduced rate of uveitis and enthesitis compared to the DMARD/NSAID group and a 50%/80% lower risk of enthesitis/uveitis as a first occurrence.36
In a separate analysis, Bessette et al. found that 12.4% of the adalimumab group compared with 24.1% of the non-biologic group switched treatments over 24 months and that overall disease burden reductions related to functional capacity and self-reported disease activity were greater in the adalimumab group, with a greater chance of a therapeutic response and shorter time to achieve this compared to placebo. However, after 24 months, there was no difference in residual disease burden.37
Kinase Inhibitors
As can be seen from Figure 1, JAKi are now recommended treatments for several PsA symptoms.28 The JAKi upadacitinib was shown in the 12/24-week Phase III SELECT-PsA 1 and 2 trials to be an effective drug for people with PsA.38,39 Atul Deodhar et al. analysed this data with a view to characterising patients with PsA with and without axial involvement and comparing upadacitinib and placebo cohorts. They found that while disease burden at baseline was higher in patients with PsA with axial involvement, 15 or 20 mg upadacitinib was still effective in improving axial symptoms compared to a placebo over 12 and 24 weeks.40 The 2/3 SELECT-AXIS trial was a similar study by Deodhar et al. in patients with radiographic AxS. Here, they found a sustained and consistent efficacy with no new safety signals in patients receiving 15 mg upadacitinib for 50−64 weeks.41
Another analysis by this group, presented by Xenofon Baraliakos et al., included both SELECT-PsA and SELECT-AXIS data. They found that those with axial PsA had higher peripheral joint and psoriasis burden compared with patients with AxS and peripheral involvement, but that this latter group had significantly greater overall pain and back pain scores than those with axial PsA. Regardless of axial involvement or diagnosis, 15 mg upadacitinib was found to be as effective for all groups with similar improvements versus placebo.42
Baraliakos et al. also presented an investigation of the JAK-1 preferential inhibitor filgotinib, as carried out on patients with radiographic AxS as part of the Phase II TORTUGA trial. Post-hoc evaluation of inflammatory and fat spinal lesions found that total spine inflammation score significantly reduced in those taking filgotinib for 12 weeks compared to placebo; however, of note, inflammation scores were higher than placebo at baseline. Specific locations where inflammation decreased include posterior elements, facet joints, and the spinal vertebral body region.43
Deucravacitinib (formerly BMS-986165) is an oral, novel selective tyrosine kinase 2 inhibitor that inhibits key cytokines involved in PsA such as IL-23.44 Philip Mease et al. presented data from a 16-week Phase II trial of 6 mg/day (n=70) or 12 mg/day (n=67) deucravacitinib for PsA versus a placebo.45 Key findings were significant differences in overall response compared with placebo, with also significant differences by dose, with a greater number on the 12 mg/day dose achieving a response. Responses were irrespective of body weight or prior TNFi use. More specific analysis of response found both patient and physician assessments of disease activity to have a greater change from baseline in those in the deucravacitinib groups, along with reduction in CRP levels and swollen and tender joint counts. Analysis of enthesitis resolution found significant advantages for deucravacitinib but, while dactylitis resolution was greater with deucravacitinib, it was not significantly different from the placebo. There were no drug-related serious adverse events, with only one occurring in the placebo group, and no deaths. Treatment-related adverse events were recorded in 31.4%/25.4% of those receiving 6/12 mg deucravacitinib and 9.1% of those receiving the placebo. The authors concluded that deucravacitinib was efficacious versus placebo with the safety profile consistent with that found in previous studies.45,46
Interleukin
The ILi secukinumab is being investigated in the ongoing, prospective, non-interventional SERENA study. Interim analysis by Uta Kiltz of 534 PsA and 470 radiographic patients with AxS found 1/2-year retention rates of 85.2%/74.9% in those with PsA and 85.8%/78.9% for AxS. Investigating dosing, at baseline, they found that most of those with PsA (79.5%) received 300 mg secukinumab and most with AxS (97.0%) received 150 mg secukinumab. After 2 years of treatment, most patients in both groups continued with their initial dose. A treatment break was given to 31 patients with PsA (median duration: 125 days) and 42 patients with AxS (median duration: 118 days), due to adverse events in 58.1% and 45.2%, respectively.47
IL-17 is known to have a great involvement in the pathogenesis of radiographic AxS.1 The humanised IgG1 monoclonal antibody netakimab targets IL-17A and Inna Gaydukova et al. presented a post-hoc analysis of the Phase III BCD-085-5/ASTERA study, which compared 120 mg netakimab with a placebo for 16 weeks in patients with radiographic AxS. They found a decline of disease activity in the netakimab group irrespective of baseline sacroiliitis.48
Diagnosis, disease activity, and treatment disparities have been found in females with AxS.49 Accordingly, sex differences were investigated by Irene van der Horst-Bruinsma et al. in a trial of ixekizumab for AxS. They found that, at baseline, female patients were older and had significantly higher peripheral joint symptoms and fatigue and pain scores. The response rate to 80 mg ixekizumab every 2 weeks in those with radiographic AxS (males: n=298; females: n=78), was, at Week 16, 39.0% in males and 16.7% in females and, at Week 52, 44.0% in males and 33.3% in females. In non-radiographic AxS (males: n=99; females: n=99), respective figures for males/females were 46.0%/23.9% at Week 16 and 30.0%/30.4% at Week 52. They concluded that while both males and females benefitted from ixekizumab, this was after a longer period of treatment administration in females.50
The PsABio study was a prospective, observational study in people with PsA that evaluated persistence, effectiveness, and tolerability of ustekinumab versus a first-, second-, or third-line TNFi administered for up to 3 years.51 To examine sex differences in burden of PsA and treatment persistence at 12 months (±3 months), van der Horst-Bruinsma et al. presented a sub-analysis of this data, including 494 females and 399 males.52 At baseline, while age and disease duration was similar, females had worse scores of disease activity and severity, disease-related health assessment, and comorbidities, including cardiovascular/metabolic syndrome, obesity, and anxiety/depression. They were also more often on third-line DMARD treatment. Significantly higher baseline tender joint counts compared with males was reflected in 12-month data for females. For enthesitis, while baseline scores were not significantly different, 12-month scores were significantly much lower in males than females and while enthesitis resolved in 75% of males, this was only shown in 46% of females. Overall, while composite endpoints were high, they were lower in females. Perhaps, due to these findings, significantly more females than males discontinued or switched treatment, and at an earlier timepoint, with similar findings for both drugs. The authors concluded that “more comprehensive treatment before severe disease manifestations evolve may improve disease management in female patients with PsA.”52
Other Treatments
One novel treatment for a number of inflammatory conditions is faecal microbiota transplantation.53 Maja Skov Kragsnaes et al. carried out a study of such for PsA53 and here reported that patients who participated in their randomised, controlled trials found the treatment acceptable and safe, which Kragsnaes et al. suggested encouraged more research into faecal microbiota transplantation.54
Conclusion
Overall, at the EULAR 2021, there was a callout for better understand regarding the pathophysiology of AxS and PsA; earlier recognition and diagnosis; a T2T approach; and a holistic view of disease burden, as has been backed up by several studies and guidelines.18,55-57
The presentations discussed here highlighted an unmet need to more fully investigate specific symptoms of AxS and PsA and decrease diagnostic delay, as well as showing how studies of treatment for these symptoms bring the hope of more tailored treatment for people with AxS and PsA.








