Meeting Summary
Prof McGonagle introduced the symposium and briefly described the aims of the meeting. Dr Behrens first discussed how findings from relevant psoriasis and psoriatic arthritis (PsA) registries can be applied to improve daily practice, and reflected on the real-life effectiveness of biologic therapies in the treatment of PsA. Prof McGonagle then followed with a discussion describing the key immunological pathways involved in psoriasis and PsA, evaluating the key similarities and differences in tissue and cytokine pathobiology in both conditions. Prof Danese then concluded the symposium by presenting on the pathophysiology of the interleukin (IL)-23 pathway in inflammatory bowel disease (IBD), reviewing the latest data for IL-23 inhibitors in treating IBD.
Comparing Interleukin-12/23 and Anti-Tumour Necrosis Factor ‘Real-World’ Registry Data in Psoriasis and Psoriatic Arthritis
Doctor Frank Behrens
Defining and adhering to therapeutic targets have been shown to be critical for improving treatment outcomes across a number of diseases, including diabetes, hypertension, and rheumatoid arthritis. For spondyloarthropathies (SpA), such as PsA, the treatment target has been defined as remission or, alternatively, low disease activity.1 This treatment target can be achieved by utilising different outcome measures to formulate an appropriate personalised treatment plan suited to specific patients within clinical practice.
To achieve treatment goals in SpA, firstly the treatment target must be based on a shared decision between the patient and their rheumatologist. For example, in a randomised controlled trial (RCT) conducted by de Vries et al.,2 two biologics, infliximab (5 mg/kg intravenously at Weeks 0, 2, 6, 14, and 22) and etanercept (50 mg subcutaneously twice weekly), were compared for the treatment of psoriasis. At Week 24, significantly more patients achieved ≥75% improvement of Psoriasis Area Severity Index (PASI 75) with infliximab (72%) than with etanercept (35%; p=0.01). Additionally, infliximab demonstrated a significant reduction in body surface area (BSA) compared with etanercept. From a rheumatologist’s perspective, based on these data, infliximab would be considered the favourable treatment over etanercept. However, in the same study, patients were asked to complete a Treatment Satisfaction Questionnaire for Medication, which assessed the patient’s satisfaction with their treatments, based on adverse events, convenience, efficacy, and global satisfaction.2 Although it was not statistically significant, etanercept scored higher than infliximab with regard to convenience and adverse events, which may be due to its subcutaneous application. Surprisingly, however, there was no significant difference in efficacy satisfaction between the two treatments, despite the significantly greater PASI 75 response with infliximab compared with etanercept.2 The treatment target should therefore be based on a shared decision between the patient and their rheumatologist. If both views differ, then other goals and different data should be considered to guide the treatment decision.
It should be borne in mind that the primary goal of treating patients with SpA is to maximise long-term health-related quality of life through control of signs and symptoms, avoidance of toxicities, and minimisation of comorbidities.1 To achieve this goal, physicians can look to RCT and real-world evidence (RWE). RWE is more advantageous in guiding physicians’ treatment decisions compared to data obtained from RCT. In RWE, patient treatments are determined by doctors’ choices as per standard practice and are not randomised. In addition, non-adherent patients are able to switch treatment and so are likely to remain included in the data, whereas, in RCT, non-adherent patients are taken out of the analysis. RWE also contains a heterogeneous patient population reflecting a realistic scenario, unlike RCT, which contain a number of inclusion and exclusion criteria that artificially create a homogenous treatment group. Given these advantages over RCT, data from psoriasis and PsA registries, which better reflect the real-life effectiveness of biologic therapies, can be applied to improve daily practice.
Several studies using data from registries across the world have compared the efficacy and safety of treatment options for psoriasis and PsA. A study from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) assessed the risk of adverse events of special interest with psoriasis treatments in a real-world setting3 and found that rates of serious infection for infliximab and other biologics were numerically higher compared with ustekinumab. Similarly, a study using data from the prospective Biologisk Behandling i Dansk Dermatologi (DERMBIO) registry in Denmark demonstrated a trend of lower cumulative incidence of serious adverse events, such as infection, cancer, and cardiovascular events, with ustekinumab compared with adalimumab, etanercept, and infliximab.4 More recently, the SUSTAIN study,5 conducted in Germany, reported the efficacy, safety, and tolerability of ustekinumab for the treatment of PsA in routine clinical care from both the physician and patient perspective. At Week 16, efficacy of ustekinumab was rated excellent by 32.3% of physicians, and a further 44.8% of physicians rated it as good. Among patients, efficacy of ustekinumab was rated as excellent or good by 34.0% and 40.2%, respectively. With respect to tolerability of ustekinumab at Week 16, it was rated excellent and good by 51.0% and 43.8% of physicians, respectively, and by 55.0% and 37.0% of patients, respectively.5
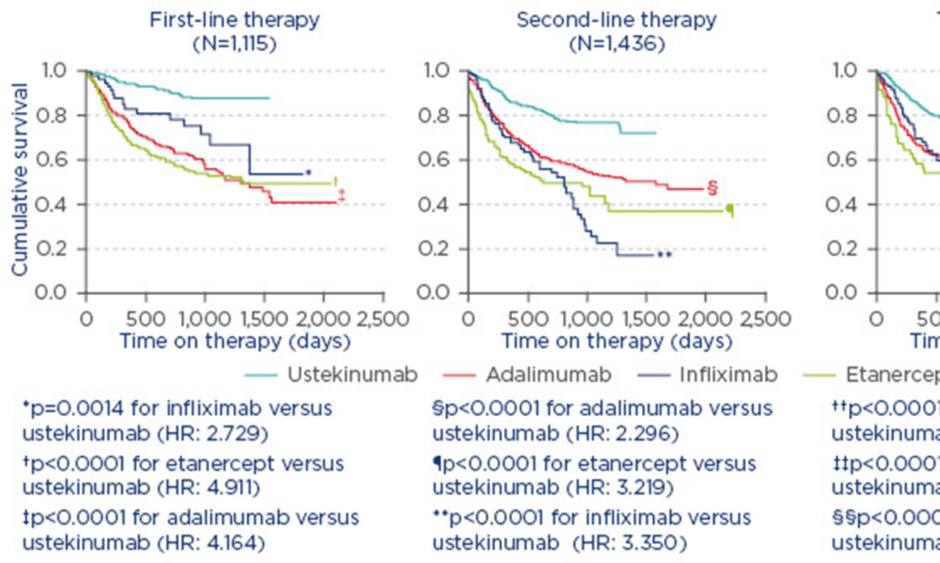
In the treatment of PsA, it is also necessary to measure disease activity and amend therapy in cases of persistently active disease.1 For example, in a study from the British Association of Dermatologists’ Biologic Interventions Register (BADBIR) which assessed drug survival (time to drug discontinuation) of biologics used to treat psoriasis, ustekinumab was shown to have the highest first-course drug survival as compared with adalimumab, etanercept, and infliximab.6 Similarly, a study from the DERMBIO registry demonstrated that ustekinumab had a significantly longer survival rate than other anti-tumour necrosis factor-alpha (TNF-α) agents.4 In another study, from the PSOLAR registry, which compared drug survival in patients with psoriasis undergoing first, second, or third-line treatment with ustekinumab, infliximab, adalimumab, or etanercept, significantly shorter times to discontinuation were observed for infliximab (hazard ratio [HR]: 2.73; 95% confidence interval [CI]: [1.48–5.04]; p=0.0014), adalimumab (HR: 4.16; 95% CI: 2.80–6.20; p<0.0001), and etanercept (HR: 4.91; 95% CI: 3.28–7.35; p<0.0001) compared with ustekinumab (reference treatment) for first-line biologic use.7 Similar results were observed for second and third-line therapies (Figure 1).7
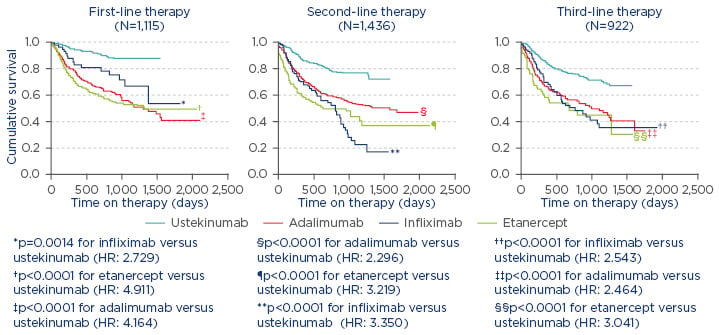
Figure 1: Proportion of patients continuing therapy amongst known biologic therapies in psoriasis.7
Reasons for stop/switch were similar across biologics.
HR: hazard ratio.
In addition to RWE providing physicians with information to guide treatment decisions, it is also important to consider the patient perspective. Taken together, these results demonstrate that optimising treatment scores is not the same as optimising treatment and that outcome measures, along with clinical data, should be carefully taken into consideration when formulating an appropriate personalised treatment plan for patients in clinical practice.
In conclusion, the primary goal in treating psoriasis and PsA is to lower disease activity and maximise long-term health-related quality of life. Real-life data from psoriasis and PsA registries provide useful information and can be used to make informed treatment decisions. Furthermore, outcome measures should be taken into consideration and treatment targets should be based on a shared decision between the patient and their rheumatologist, since optimising treatment scores does not always translate to optimising treatment.
Immunological Similarities and Differences Between Psoriasis and Psoriatic Arthritis
Professor Dennis McGonagle
Understanding the clinical relevance of enthesitis and the immunology at the enthesis has helped to understand similarities and differences between psoriasis and PsA. Enthesitis is the inflammation of the enthesis and is well recognised in the synovial joints of patients with PsA.8 Recent findings have shown that the immunopathogenetic inter-relationship between the synovium and entheses is more complex than first imagined and that joint-specific factors found in the synovio-entheseal complex could trigger innate immune responses and may be pivotal players in the phenotypic expression of PsA.8
PsA has a wide spectrum of disease severity. The clinical heterogeneity in PsA reflects the substantial genetic heterogeneity. In recent years, many genes that contribute to the pathogenesis of psoriasis and PsA have been identified.9 As an example, genetics studies have demonstrated that polymorphisms in the TNF-α interactive protein 3 (TNFAIP3) gene, which encodes the ubiquitin-modifying protein, A20, an important endogenous regulator of inflammation, are linked to both psoriasis and PsA10 and play a role in the development of enthesitis, with the earliest manifestation occurring within the synovio- entheseal complex resident myeloid cells.11
In recent years, several animal models have been developed that demonstrate the primacy of enthesitis in SpA-like disease. The most exciting translational model is that of arthritis following systemic overexpression of IL-23 in the liver, which resulted in three cardinal manifestations, namely a primary enthesis, skin rash, and aortic root inflammation.12 In this particular model, populations of innate lymphoid-like cells (ILC) were documented at the entheses and these were key to driving the disease process.
In humans, Group 3 ILC (ILC3) play a pivotal role in barrier tissues, such as the gut and the skin, two important sites of disease in SpA. A recent study has demonstrated that there is a higher proportion of ILC3 in human entheseal soft tissue compared with peripheral blood (p=0.008), and a similarly higher proportion of activated ILC3 in both entheseal soft tissue and peri-entheseal bone (p=0.01 and p=0.043, respectively, versus peripheral blood).13 Furthermore, normal entheseal digests stimulated with IL-23/IL-1β upregulated IL-17A transcripts and histological examination of injured/damaged entheses showed retinoic acid receptor-related orphan receptor gamma (RORγ)-expressing cells.13
Recent studies in the aforementioned IL-23-dependent murine model have shown that many of the entheseal resident IL-17-producing cells were gamma delta (γ-δ) T cells.14 This family of innate immune lymphocytes is best recognised for localisation at sites of barriers where stress and injury is common, allowing them to sense damage;15 they are now recognised as key players in the pathogenesis of IL-23-induced entheseal inflammation. It was recently discovered that γ-δ T cells are present in normal human enthesis and that they constitute a greater proportion of the T cell pool in entheseal soft tissue compared with peripheral blood, making it likely that they represent a tissue-resident population.16 This is the first description of γ-δ T cells at the human enthesis and offers tentative confirmation of findings in mouse models where these cells play a key role in SpA pathogenesis. This is the first confirmation in humans that across the SpA spectrum of disease, from gut to skin to enthesis, IL-23-dependent innate immune cell populations are present.
RWE has provided a proof-of-concept of the key importance of the IL-12/IL-23 axis in PsA. The PHOENIX 1 study,17 which examined the long-term efficacy and safety of ustekinumab through 5 years of continuous treatment, demonstrated that initial clinical responses were generally maintained through to Week 244 (PASI 75: 63.4% and 72.0% for patients receiving 45 mg and 90 mg, respectively) (Figure 2).
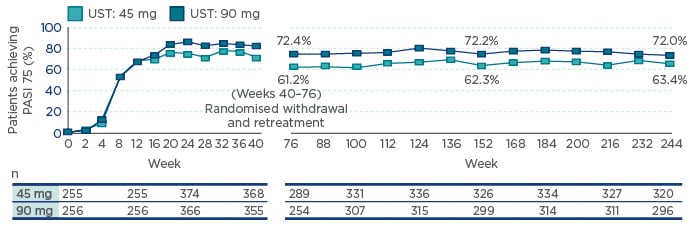
Figure 2: The PHOENIX 1 study: The proportion of patients achieving ≥75% improvement in Psoriasis Area Severity Index efficacy in the overall population through 5 years.
Note: Placebo crossover patients were included at the beginning of Week 24 (i.e. 12 weeks after UST treatment). Analyses were not conducted between Weeks 40 and 76 when the majority of the population was withdrawn from treatment per study design. Analyses resumed at Week 76 when about half of the withdrawn patients had reinitiated UST for ≥12 weeks. Patients who reinitiated treatment after Week 76 were reincluded after ≥12 weeks of retreatment. UST 90 mg is indicated for patients with a body weight of >100 kg.
PASI 75: 75% improvement in Psoriasis Area Severity Index; UST: ustekinumab.
Subclinical enthesopathy has also been shown to be a commonality between psoriasis and PsA. Ultrasonographic enthesopathy was present in 11.6% of entheses in the psoriasis group and 5.3% of entheses in the control group (p<0.0005).18 Furthermore, treatment with ustekinumab led to an improvement in subclinical enthesopathy in patients with psoriasis.19 The data suggest that early intervention with anti-IL-12/IL-23 agents in psoriasis may prevent the evolution of subclinical disease into frank PsA. However, this needs to be evaluated in real-world registry data sets to confirm that PsA evolution can be prevented when the IL-23/IL-17 axis is targeted. In summary, there are strong common genetic and immunological overlaps between psoriasis and PsA; however, these diseases are highly heterogeneous. Emerging data in both preclinical and clinical studies have identified different populations of innate lymphocytes that are critically dependent on the IL-23/IL-17 axis.
The Link Between the Joint and the Gut: A Gastroenterologist’s View
Professor Silvio Danese
The link between the joint and the gut was explored from a gastroenterologist’s perspective through a patient case study; the patient was female and 48 years old. She had had guttate psoriasis since she was 25 years of age and developed PsA, which affects both knees, 6 years before the study. She was treated with non- steroidal anti-inflammatory drugs and methotrexate at 10 mg/weekly, which was then reduced to 7.5 mg/weekly. She presented with abdominal pain and diarrhoea (4–6 stools/day, although not at night), with mucus but no blood in her stool. Her histopathology results confirmed the diagnosis of Crohn’s disease and she was administered steroids and methotrexate (25 mg/weekly). She responded well to her treatment, later continuing on methotrexate and tapering off of the steroids. In April 2015, she complained about joint pain and sleep disturbances; she stopped her methotrexate treatment and was started on adalimumab by her rheumatologist.
By July 2015, the patient’s condition had worsened. She had diarrhoea with blood and mucus in her stool, her calprotectin level was 3,800 µg/g, and her C-reactive protein was 5 mg/dL. Her psoriasis worsened and required local treatment, and she developed scleritis, which was controlled by topical steroids. Her joint pain was managed by adalimumab 40 mg/weekly.
In November 2016, a magnetic resonance enterography and an ileocolonoscopy were performed to establish the location and extent of her Crohn’s disease. The results demonstrated active bowel inflammation but no evidence of small bowel disease. Through a multidisciplinary consultation, it was decided to treat the patient with ustekinumab 6 mg/kg via an intravenous, subcutaneous dose at Week 8, and then every 12 weeks thereafter. The patient’s condition improved, with her C-reactive protein decreasing to 0.62 mg/dL and her stools reduced to 1–2 per day and were without blood. She had no abdominal pain, no psoriatic lesions, and no ulcers or narrowing of the colon.
UNITI-1 and UNITI-2 are two Phase III randomised, double-blind, controlled studies that compared the effects of a single intravenous dose of ustekinumab (either 130 mg or approximately 6 mg/kg of bodyweight) to a placebo over 8 weeks in patients with moderate-to-severely active Crohn’s disease.20 The UNITI-1 trial included 741 patients who met the criteria for primary or secondary non-response to TNF antagonists or had unacceptable side effects. The UNITI-2 trial included 628 patients in whom conventional therapy had failed or unacceptable side effects occurred. Patients who completed these induction trials then participated in IM-UNITI, which evaluated the efficacy and safety of two maintenance regimens of 90 mg of ustekinumab administered subcutaneously (either every 8 weeks or every 12 weeks) versus placebo (Figure 3).
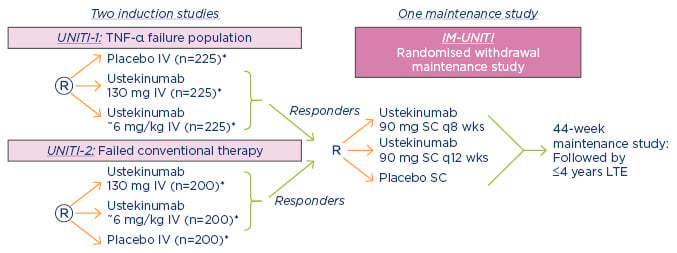
Figure 3: UNITI Phase III Crohn’s programme.
*Subjects randomised to placebo and subjects who are non-responders to ustekinumab are eligible for non-randomised maintenance dosing after completion of the induction study.
IV: intravenous; LTE: long-term extension; q8 wks: every 8 weeks; q12 wks: every 12 weeks; SC: subcutaneous; TNF-α: tumour necrosis factor-alpha.
In both UNITI-1 and UNITI-2, clinical remission, defined as a Crohn’s Disease Activity Index (CDAI) score of <150 points, was found at Week 8 to be significantly higher in both of the ustekinumab groups compared with placebo.20 In IM-UNITI, the percentage of patients who were in remission at Week 44 was significantly higher in the groups that received 90 mg of ustekinumab every 8 weeks or every 12 weeks than in the placebo group, with an absolute difference between treatment every 8 weeks and placebo of 17.2 percentage points, and a difference between treatment every 12 weeks and placebo of 12.9 percentage points.20
From a gastroenterologist’s view, there is a strong link between the joints and the gut, as extra-intestinal manifestations, such as peripheral arthropathy and arthritis, are prevalent among patients with Crohn’s disease and ulcerative colitis.21,22 Back and joint pain are the most common extra-intestinal symptoms reported by patients with IBD and can significantly lower a patient’s quality of life and work productivity.23 Most patients with IBD are aware of the risk of developing arthritis;24 however, in a study that investigated self-reported prevalence of musculoskeletal SpA features in a cohort of patients with IBD, a substantial number of patients had not been evaluated by a rheumatologist.25 This is therefore an area where gastroenterologists and rheumatologists can work more closely together to improve the treatment of immune-mediated inflammatory diseases. In clinical practice, it would be useful to define the red flags that will help clinicians to make a correct diagnosis of IBD-associated SpA.26 Diagnostic clues for rheumatologists to consider include family history of IBD; clinical symptoms such as clinical diarrhoea, chronic abdominal pain, and weight loss; and history or evidence of perianal fistula/abscess and anaemia. For gastroenterologists, the following diagnostic clues should be considered: chronic back pain (>3 months), peripheral joint pain/swelling, the presence of signs of enthesitis, and history or evidence of dactylitis. Early recognition of extra-intestinal manifestations will also help guide therapy and reduce overall morbidity in affected patients.27
In summary, IBD such as Crohn’s disease share a commonality with psoriasis and PsA through the IL-23 pathway. Similarly to psoriasis and PsA, clinical studies have shown that inhibiting the IL-23 pathway induces response and remission in patients with moderate-to-severely active Crohn’s disease. We should therefore consider how multidisciplinary management can be leveraged to improve the treatment of immune-mediated inflammatory disease.