Meeting Summary
Artificial intelligence (AI) describes the use of technology to mimic the cognitive functions of a human being, such as intelligent behaviour and critical thinking. AI-based technologies are already being used across healthcare settings for applications such as image analysis and diagnosis; nevertheless, the full potential of AI to guide disease management in rheumatology has yet to be realised.
Rheumatoid arthritis (RA) is a chronic inflammatory disease with a heterogeneous clinical presentation and pathogenesis. Early intervention with disease-modifying therapies can alter the course of the disease, preventing irreversible joint damage and improving long-term outcomes. However, barriers to early diagnosis of rheumatic diseases mean that many patients receive delayed treatment, leading to sub-optimal long-term outcomes. Patients at high risk of disease progression are particularly suitable for early intervention, although accurately identifying these patients can be challenging. AI approaches to aid in early diagnosis and prediction of disease progression are emerging in rheumatic diseases, but these have yet to enter clinical practice.
Prediction of response to treatments such as anti-TNFs is another area where AI could play an important role in the future. Many patients with rheumatic diseases have a sub-optimal response or loss of response to certain therapies, and methods to predict response are lacking. AI approaches for predicting treatment response are under investigation in rheumatology, and many potential biological predictors of response to anti-TNFs have been identified. However, these studies are still at an exploratory stage, and the results will need validation before they can be implemented in clinical practice.
AI approaches have the potential to transform the treatment of rheumatic diseases, from advancing early diagnosis to facilitating a more individualised treatment approach, with the overall aim of improving patient outcomes.
Introduction
This symposium was developed to consider the future role that AI might play in the management of rheumatic diseases. First, Ignacio Medrano considered the current use of AI in healthcare and its possible future applications. Ernest Choy subsequently discussed the potential role of AI in facilitating early intervention in rheumatic diseases. Finally, Laure Gossec outlined opportunities to use AI in the prediction of treatment response and considered the ethical and practical implications of using AI to analyse ‘Big Data’.
Artificial Intelligence: The Future of Medicine?
AI is a term used to describe the use of computers and other technology to simulate intelligent behaviour and critical thinking that mimic the cognitive functions of a human being.1 Research into the application of AI techniques in medicine has been ongoing for several decades; in 2016, most AI investment went into research in the healthcare sector.1 The potential applications of AI in medicine are vast and, ultimately, are likely to have significant impact on patient care and clinical decision-making in rheumatology.2
There are potential applications for AI-based technologies in almost every aspect of modern life. One well-known example of AI is the programme Google Translate (Google, Mountain View, California, USA), which can automatically translate text to and from a multitude of languages. Google Translate uses an algorithm based on machine learning, an AI technique, which is programmed to learn from a set of solved problems. This approach removes the need for any understanding of grammar or syntax and allows the algorithm to infer the rules of any language and apply them to solve unseen problems.
AI-based technologies are emerging within healthcare settings, but more research is needed before the full potential of AI is realised across a wide range of clinical applications. Automated analysis of medical imaging is one area where AI may have particular value because manual analysis of these images is resource intensive and can be subject to inter-observer variability.3 In 2016, a deep-learning algorithm was developed that detected diabetic retinopathy and macular oedema in retinal fundus images with a high degree of specificity and sensitivity.4 Based on these findings, in 2018 the U.S. Food and Drug Administration (FDA) permitted marketing of the first AI-based medical device that could be used in a primary care physician’s office to detect cases of diabetic retinopathy.5 AI techniques also have the ability to extract unexpected patterns and associations from medical images. For example, a deep-learning algorithm has been developed that can predict cardiovascular risk factors (such as age, sex, and systolic blood pressure) from retinal fundus images.6 Previously, it had not been considered that these factors could be identified from retinal images.6 This demonstrates the power of AI techniques to go beyond human interpretation to see associations between multiple variables that the human brain cannot detect.
Other applications of AI in medicine have been investigated, including the use of laboratory data, medical records, and molecular biology outputs to aid in diagnosis, treatment selection, and prediction of prognosis. For example, a model based on demographic and laboratory data was developed to predict treatment non-response in patients with Crohn’s disease.7 AI systems have also been used to assist selection of an appropriate antibiotic prescription.8 Numerous AI-based technologies have been approved by the FDA across fields including oncology, cardiology, and emergency medicine.9
The use of AI in rheumatology is not as advanced as in other therapy areas; more research is needed into the development and implementation of AI techniques to aid with diagnosis and management of rheumatic diseases. However, some promising research has been undertaken in arthritis; in particular, a study that aimed to detect early osteoarthritis by examining pre-symptomatic cartilage texture maps from MRI using an automated pattern-learning system.10 Future applications of AI in rheumatology could include examining associations between genotype and phenotype, as well as using AI to extract and analyse clinical data from electronic health records (EHR). EHRs contain large amounts of real-world patient data in both a structured form (information such as International Classification of Disease [ICD] codes), as well as in a free-text arrangement (e.g., the narrative from the healthcare provider notes).11 It can be challenging to identify or classify patients with certain conditions using structured information alone because their use can vary substantially across healthcare systems. This is a particular issue when trying to identify patients with axial spondyloarthritis (axSpA) from EHRs because the evolving disease concept means there has historically been a lack of specific ICD codes. To overcome this issue, a technique called natural language processing was used in one study to extract key disease concepts from free-text data in EHRs.11 These data were then combined with structured ICD code data to develop algorithms designed to identify patients with a high probability of having axSpA. When identifying patients with axSpA, the algorithms that incorporated free-text data outperformed the algorithms that used ICD codes alone.11 Algorithms incorporating data derived from natural language processing expand the amount of EHR data that can be analysed and offer exciting opportunities for clinical research in the future.
AI in healthcare is a rapidly evolving field, but it is important to proceed cautiously and responsibly. In a recent proof-of-concept study, a deep-learning algorithm was used to detect patients with atrial fibrillation based on facial video images.12 The researchers used the approach to identify patients with atrial fibrillation with a high degree of accuracy and could facilitate high-throughput screening in settings such as hospital waiting rooms.12 However, the non-contact nature of this type of examination raises important questions and concerns about patient consent, privacy, and confidentiality.13 As technology advances, regulation will be required to ensure that tools are used appropriately and ethically.
Can Artificial Intelligence Find the Missing Pieces Needed to Facilitate Early Intervention?
RA is a chronic inflammatory disease mainly affecting the joints but is heterogeneous in terms of clinical presentation and disease pathogenesis.14 Patients with RA have variable clinical courses characterised by continuously active disease or periods of relapse and remission.15 In RA, a ‘window of opportunity’ is thought to exist between the onset of inflammation and the start of radiographic damage. Early intervention with disease-modifying anti-rheumatic drugs (DMARDs) during this ‘window of opportunity’ aims to alter the disease course before irreversible joint damage occurs, improving long-term outcomes.15
Early diagnosis and early intervention with effective therapies remain challenges in rheumatology. A study in patients who were newly presenting with RA or unclassified inflammatory arthritis showed delays occurring at several points in the care pathway. The median time between symptom onset and seeing a rheumatologist was 27.2 weeks, with only 20% of patients being seen within 3 months of symptom onset.16
Diagnostic Delay in Rheumatology
A number of potential barriers to early diagnosis in RA have been identified, including a broad differential diagnosis, heterogeneous presentation at early stages of disease, differences in symptom onset, and the fact that no single laboratory test is specifically characteristic of RA.16,17 Delayed diagnosis is not just a problem in RA. For patients with ankylosing spondylitis (AS) and psoriatic arthritis, an average delay of ≥8 years between symptom onset and disease diagnosis has been reported.18 Earlier diagnosis of rheumatic diseases is needed to ensure patients begin treatment before irreversible structural damage occurs.
AI approaches to improve diagnosis are being investigated in rheumatology (Figure 1). In one study, machine learning was used to develop an algorithm to identify patients with RA from EHRs.19 Eight diagnosis or medication codes within the EHRs were found to be associated with a diagnosis of RA and were used to build the final model. The resulting algorithm had an accuracy of 92.3% for identifying patients with RA, comparable with expert clinical knowledge-based methods.19 In a separate study, an artificial neural network model was constructed that could classify patients as having RA, osteoarthritis, or being non-arthritic, based on differentially expressed serum cytokines. The resulting model was able to accurately diagnose 100% of test patients correctly.20
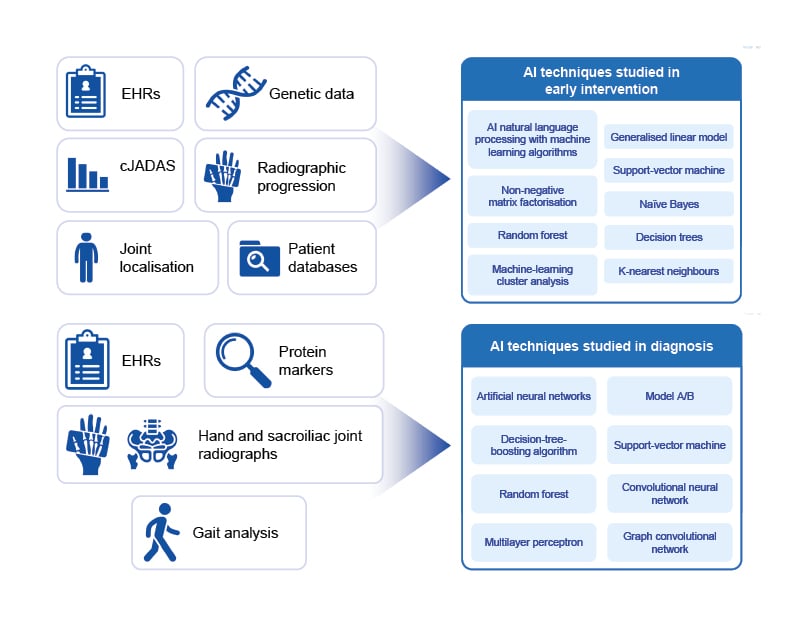
Figure 1: Artificial intelligence techniques studied in diagnosis and early intervention in rheumatic diseases.
AI: artificial intelligence; cJADAS: clinical Juvenile Arthritis Disease Activity Score; EHRs: electronic health records.
Early Intervention
European Alliance of Associations for Rheumatology (EULAR) treatment guidelines for RA recommend that in patients with an inadequate response to first-line conventional synthetic DMARDs and poor prognostic factors, a biologic DMARD or a JAK inhibitor should be added to the initial treatment strategy.21 However, EULAR treatment recommendations are not always followed in clinical practice; a real-world study of 2,536 patients in Europe revealed that 55.4% of patients eligible to receive biologic DMARDs were not receiving them.22
AI approaches have been investigated to help identify patients with rheumatic diseases at high risk of progression who are particularly suitable for early intervention (Figure 1).
In patients with axSpA, machine-learning models were used to classify patients into three distinct groups based on clinical characteristics.23 Radiographic progression was assessed over 2 years and was found to differ significantly between the three groups. As joint damage is irreversible, a model that can accurately identify patients at high risk of radiographic progression who would benefit from early aggressive treatment would be of great benefit in rheumatology.
Similarly, AI has been used to identify factors predictive of which patients with AS may require early use of anti-TNFs.24 An artificial neural network model combined demographic and laboratory data from 595 patients with AS who were grouped according to early anti-TNF use or not. In the test data set, the model was able to predict which patients would require anti-TNF treatment within 6 months of diagnosis more accurately than conventional statistical methods. The model also identified the acute phase reactants C-reactive protein and erythrocyte sedimentation rate as important prognostic factors of early anti-TNF use.
RA is known to be heterogenous in nature from the time of diagnosis, and the molecular and cellular signatures associated with disease progression and therapeutic response are beginning to be understood.25,26 Identification of factors that predispose a patient to a rapidly progressive disease course could inform AI models to identify patients who require early intervention.
Predicting Response to Treatment: Is Artificial Intelligence the Next Piece of the Puzzle?
AI approaches are evolving in rheumatology (Figure 2). Research has progressed from initial exploratory proof-of-concept studies, through to the development of time-saving AI-based tools, such as algorithms capable of automated image analysis. Researchers are starting to investigate algorithms to help guide clinical decisions, but we have still not reached the overall goal of AI, which is to help facilitate a fully personalised treatment approach to improve patient outcomes.
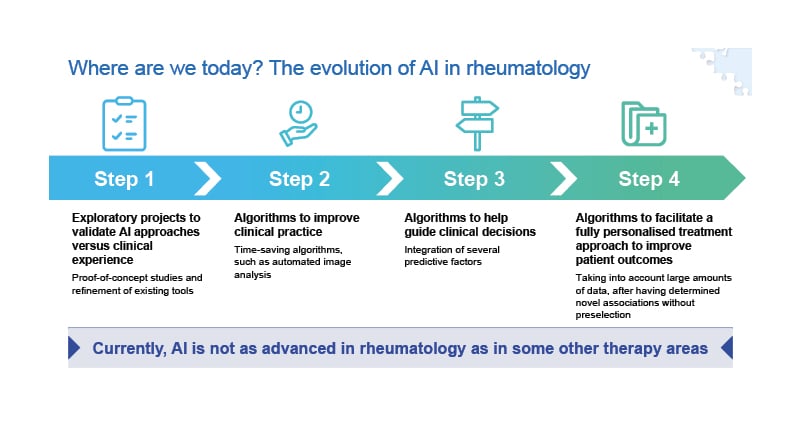
Figure 2: The evolution of artificial intelligence in rheumatology.
AI: artificial intelligence.
As many patients have a primary non-response, partial response, or secondary loss of response to certain treatments,27-29 the identification of factors predictive of treatment response could help ensure patients receive the most suitable therapy to improve outcomes and avoid disease flares. AI approaches for predicting response to treatment are under investigation in patients with RA, including prediction of anti-TNF response based on integration of clinical and genetic markers, identifying anti-TNF non-responders using a biomarker panel, and predicting anti-TNF response using multiomics.30-32
Biomarkers for Prediction of Response
Many biomarkers already play a role in the diagnosis and management of RA,33-35 and while a plethora of biomarkers that may predict response to anti-TNFs are currently being investigated, most are yet to be validated.
In an exploratory study in patients with RA, the ability of an AI algorithm based on a panel of biomarkers to identify anti-TNF non-responders was assessed. Machine learning was employed to generate a predictive algorithm to identify patients treated with anti-TNFs who would not achieve clinical response.31 The algorithm was based on the top 25 features identified from a panel of gene-expression biomarkers, single-nucleotide polymorphism, and clinical data. Features were assessed in two patient cohorts prior to treatment with an anti-TNF, and clinical response was recorded. The algorithm was subsequently tested on a validation cohort and demonstrated good predictive power for identifying patients who did not respond to anti-TNF, with a positive predictive value of 89.7% and a specificity of 86.8%.31
Electronic Health Data for Prediction of Response
In addition to the use of biomarkers, electronic health data from wearable devices also have the potential to predict response to treatment in patients with rheumatic diseases. Traditional electronic-health platforms that collect patient-reported outcomes, such as online questionnaires, place a burden on patients because they are required to actively enter information on a regular basis. Patient engagement with these platforms is known to reduce over time, especially if patients do not receive feedback on the data they enter.36 Passive data collection, such as activity or sleep data collected using a wearable activity monitor, may be a less burdensome and more reliable way to collect data on patient wellbeing. In the ActConnect study, the potential association between physical activity assessed passively using an activity tracker and disease activity was evaluated in patients with RA or axSpA. Persistent flares in RA or axSpA were found to be associated with a lower daily step count (p=0.03).37
Using machine-learning techniques to analyse patient data collected from wearable devices may allow for remote monitoring of disease activity, with a high degree of accuracy and minimal burden to the patient. A pilot study of 155 patients used machine learning to assess the association between patient-reported flares and steps per minute measured using an activity tracker over a period of 3 months. A total of 224,952 hours of physical activity assessments were analysed during the study and the model accurately predicted disease flare with a sensitivity of 95.7% and specificity of 96.7%.38 Out of 4,030 weekly flare assessments, 880 patient-reported flares were predicted by machine learning, compared with only 40 patient-reported flares that were not predicted by machine learning.38 In the future, the use of AI to monitor disease activity may offer the potential for treatment optimisation before disease flare occurs.
These studies demonstrate the promising impact of AI in rheumatology; however, more research is needed to realise the ultimate aim of using AI to offer a fully personalised treatment approach to improve patient outcomes.
Ethical Considerations for the Use of Artificial Intelligence and Big Data
In 2020, EULAR provided guidance on the collection, analysis, and use of extremely large datasets (known as Big Data) that may be analysed using AI in rheumatology (Figure 3).2 EULAR recognises that Big Data provides unprecedented opportunities to transform rheumatological research and practice, with the principal aim of improving patient outcomes. However, there are multiple challenges associated with the use of Big Data in rheumatology, including issues related to privacy, confidentiality, identity, and transparency.39
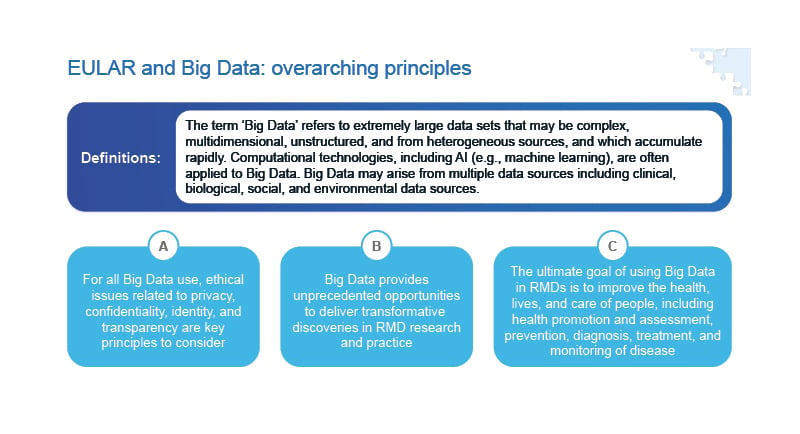
Figure 3: EULAR points to consider for the use of Big Data in rheumatology.2
AI: artificial intelligence; EULAR: European Alliance of Associations for Rheumatology; RMD: rheumatic and musculoskeletal disease.
During collection and storage of Big Data, ethics, heterogeneity of data, and data access need to be considered. EULAR has recommended that General Data Protection Regulations (GDPR) are adhered to in the European Union (EU), data are standardised, and that open data platforms are used to combat data heterogeneity and subsequent access.39 During analysis and interpretation of Big Data, EULAR has recommended multi-disciplinary learning and collaboration to ensure that methods are compared and validated while expertise and experience grow within the field of rheumatology.2
Concluding Remarks
The potential applications of AI in healthcare are vast. In rheumatology, AI has the potential to facilitate earlier diagnosis and treatment and predict individual patient response to specific therapies. This move towards precision medicine could revolutionise patient outcomes, with patients receiving individualised treatment early in the disease course, thus minimising or even preventing irreversible inflammatory damage.
Despite this huge potential, AI is in its infancy in rheumatology. Preliminary data suggest that clinical, radiographic, and biologic measures may allow rheumatologists to stratify patients according to risk of disease progression. Similarly, many potential biomarkers predictive of treatment response have been identified, but they are yet to be validated. Further studies are required to refine and validate AI approaches before they can be used to guide the management of rheumatic diseases in the clinic.
In the future, it is likely that AI will help to facilitate an individualised treatment approach in rheumatology to allow optimal disease management and patient outcomes.








