Chairperson: Georg Schett1
Presenters: Laura C. Coates,2 Kristian Reich,3 Georg Schett1
1. Universitätsklinikum Erlangen, Friedrich-Alexander University Erlangen-Nürnberg, Erlangen, Germany
2. Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences (NDORMS), University of Oxford, Oxford, UK
3. Institute for Health Services Research in Dermatology and Nursing, University Medical Center Hamburg-Eppendorf and Skinflammation® Center, Hamburg, Germany
Disclosure: Dr Coates has received research funding or honoraria from AbbVie, Amgen, Biogen, Celgene, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Prothena, Sun Pharma, and UCB. Prof Reich has been an advisor and/or paid speaker for and/or participated in clinical trials for AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Centocor, Covagen, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline, Janssen-Cilag, Kyowa Kirin, LEO Pharma, Eli Lilly and Company, Medac, Merck Sharp & Dohme, Miltenyi Biotec, Novartis, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, and Xenoport. Prof Schett has participated in speakers bureaus for AbbVie, BMS, Celgene, Eli Lilly and Company, Janssen, Novartis, and UCB.
Acknowledgements: Medical writing assistance was provided by Megan Breuer of Excerpta Medica, Amsterdam, the Netherlands.
Support: The symposium and publication of this article were funded by Janssen. The views and opinions expressed are those of the speakers and not necessarily of Janssen.
Citation: EMJ Rheumatol. 2020;7[1]:22-31.
Meeting Summary:
This Janssen-sponsored live symposium “Advancing the Treatment of PsA: Focus on the IL-23 Pathway,” took place virtually at the European League Against Rheumatism (EULAR) 2020 E-CONGRESS. The presentations focussed specifically on the role of IL-23 in psoriatic arthritis (PsA), including an overview of PsA pathogenesis, updates on the latest treatments, and how insights from recent clinical trials can be applied as individualised treatments in daily clinical practice for patients with PsA.
Prof Reich discussed the role of the IL-23 pathway in psoriatic skin inflammation, highlighting how
IL-23 inhibition results in high levels of clinical response in the majority of treated patients with psoriasis and the importance of early treatment for maximal disease modification. Studies are currently ongoing to examine the efficacy of IL-23 inhibition as a treatment option for patients with PsA, and it is likely that IL-17 and IL-23 both have differential roles in the psoriasis and PsA disease domains.
Analyses of clinical trial data, presented by Prof Schett, show that treatment with IL-23 inhibitors results in improved outcomes in patients with PsA, including American College of Rheumatology (ACR) and Psoriasis Area Severity Index (PASI) responses. The results of the DISCOVER-1 and -2 trials with guselkumab show that treatment significantly improved PsA outcomes, including ACR20 responses and resolution of enthesitis and dactylitis, compared with placebo in patients with PsA, solidifying the role of the IL-23 pathway in musculoskeletal diseases.
Dr Coates shared her experiences in optimal patient treatment approaches in PsA, highlighting the heterogeneous nature of the disease and sharing current treatment recommendations. She stressed the importance of personalised, treat-to-target treatment strategies, based on patient needs and disease domains, for optimal PsA management.
A Fresh Look at the IL-23 Pathway in Psoriatic Disease
Professor Kristian Reich
The Pathophysiology of Inflammatory Skin Diseases
Psoriasis is one of the most common inflammatory skin diseases, characterised by hyperproliferation and abnormal differentiation of epidermal keratinocytes, thereby initiating a chain of reactions resulting in skin lesions containing immune infiltrate T cells and dendritic cells.1 Surface markers such as cluster of differentiation 11C (CD11C), a marker for dendritic cells, and CD3, a marker for T cells, are also colocalised in psoriatic skin.1 Traditional models of psoriasis postulate that there is a delicate cross-talk between dendritic cells and T cells; cytokine signalling also drives T-cell activation, resulting in overproduction of antimicrobial peptides and cytokines, including IL-12, IL-23, IL-17, and TNFα, among others.2 However, while previous studies have indicated that IL-23 plays a critical role in psoriasis pathophysiology, there is little evidence indicating that the same is true for IL-12.3 Psoriatic lesions contain raised levels of genes encoding for both the p19 and p40 subunits of IL-23, compared with genes associated with the p35 unit of IL-12, providing a rationale for the efficacy of IL-23 blockade as a treatment for patients with psoriasis.3 Studies with briakinumab and ustekinumab, both of which bind to the p40 subunit of IL-12/23, show that treatment results in significantly improved PASI 75, 90, and 100 scores compared with placebo after 12 weeks of treatment.4-6
Previous studies have also indicated the role of IL-17 in psoriasis development, showing that increases in IL-17A, IL-17C, and IL-17F create a positive proinflammatory feedback loop in patients with psoriasis.7 Furthermore, one study found a significant correlation between clinical disease activity, measured via PASI scores, and IL-17A and IL-17F levels in patients with psoriasis.8 This same study also found that different concentrations of TNFα, IL-17A, and IL-17F were capable of synergistic activation of the antimicrobial peptide human β-defensin-2 production in keratinocytes.8 Preclinical experiments examining the roles of IL-17A and IL-17F in chronic tissue inflammation found that IL-17F functions as a key driver in chronic tissue inflammation, and that neutralisation of both IL-17A and IL-17F resulted in suppression of inflammation and favourable PsA outcomes.9
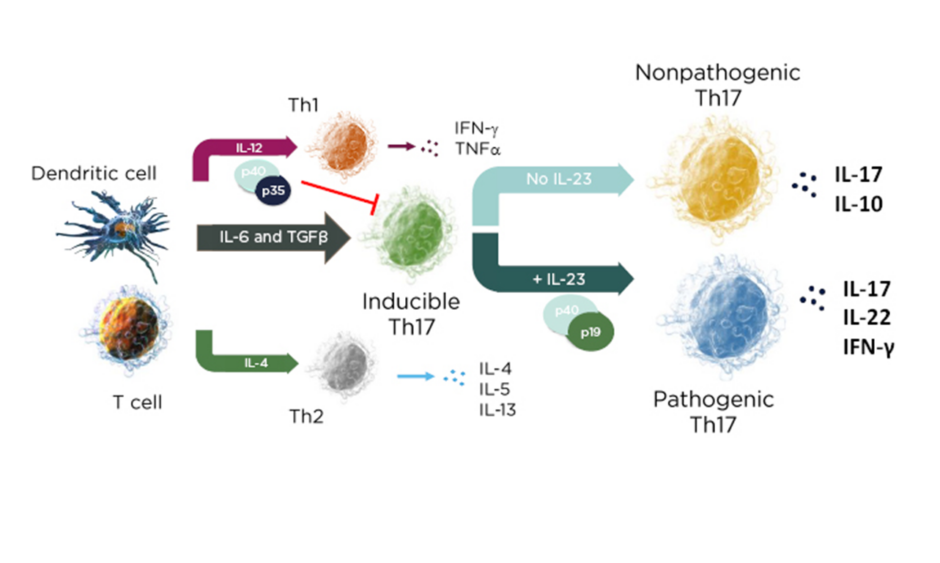
In a mechanistic study of skin inflammation, IL-17 and TNFα activation resulted in the production of osteoclast progenitor cells and multinucleated cells with increased expression of NF-ATc1, which plays a role in RANKL signal transduction and has a direct impact on bone resorption in patients with psoriasis.10 Therefore, the current psoriasis disease model contains both feed-forward and feed-backward responses, showing how the activation, upregulation, and proliferation of T cells creates a “vicious circle” of increased synergistic proinflammatory effects, increases in innate immunity, and keratinocyte proliferation.11 In the skin, the cytokine environment regulates leukocyte differentiation into functional subsets; when IL-23 levels increase, inducible IL-17 activation results in the production of pathogenic Th17 cells, while an absence of IL-23 results in the production of the nonpathogenic variant (Figure 1).12,13
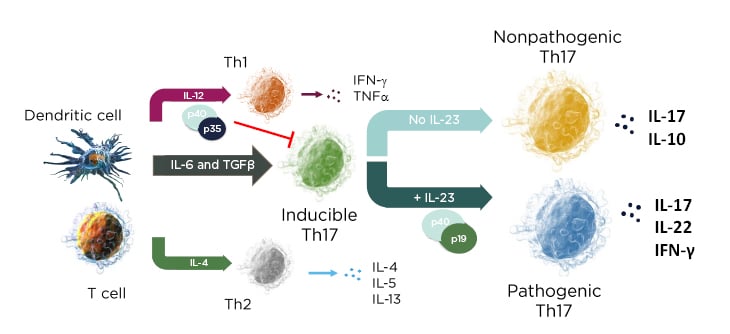
Figure 1: The cytokine environment can regulate lymphocyte differentiation into nonpathogenic and pathogenic subsets.
Adapted from Leung et al.12 and Zhu et al.13
Long-Term Disease Control and Disease-Modifying Potential of IL-23 Blockade
The development of T cells with a tissue-resident memory T (TRM) cell phenotype creates an “inflammatory memory” that develops over time in psoriatic skin. These TRM levels can remain elevated after treatment cessation in patients with clinically nonactive psoriatic lesions, indicating that IL-17 production and possible disease recurrence is maintained by TRM “disease memory” cells.14,15 Therefore, the targeting of TRM cells may be crucial in achieving long-term disease modification in patients with psoriasis.
In the VOYAGE-2 trials with guselkumab, a monoclonal antibody that specifically binds to the p19 subunit of IL-23, 88.6% and 86.0% of patients with moderate-to-severe psoriasis maintained PASI 90 responses at Weeks 48 and 72 of treatment, respectively. However, this study also showed that 36.8% and 11.5% of patients, respectively, also maintained PASI 90 responses after withdrawal from treatment, indicating that treatment resulted in long-term maintenance of response and a prolonged effect on IL-17-producing cells.16-18 Maintenance of PASI 90 responses after withdrawal was associated with continued suppression of IL-17A, IL-17F, and IL-22. Guselkumab treatment resulted in cytokine levels that were comparable to healthy controls after 20 weeks of retreatment following withdrawal, while loss of PASI 75 response was associated with increases in serum IL-17A, IL-17F, and IL-22.17
The ECLIPSE trial examined the clinical and molecular differences associated with IL-23p19 and IL-17A inhibition with guselkumab and secukinumab, respectively, in patients with psoriasis. Results showed that PASI 90 scores were maintained in 84% of patients receiving guselkumab and 70% of patients receiving secukinumab.19 An examination of T-cell frequency in psoriatic lesions and psoriatic skin showed that guselkumab treatment significantly reduced the frequency of CD8+ TRM within T cells at Week 24 of treatment, compared with secukinumab.20 Treatment resulted in reductions in TRM frequency and may subsequently halt the inflammatory cycle, indicating a possible disease-modifying effect.20 The frequency of regulatory T cells, which play a role in immune-response suppression, was maintained with guselkumab and reduced with secukinumab at Week 24 of treatment.20 Furthermore, the results of the VOYAGE-1 trial indicated that guselkumab treatment resulted in substantial proportions of patients maintaining PASI 90 responses from Weeks 52 to 204, further underlining the stable long-term responses associated with guselkumab treatment.21
In conclusion, Prof Reich emphasised the key role played by IL-23 in psoriatic skin inflammation, and that inhibition of IL-23 appears to be a safe therapy for patients with psoriasis. Treatment results in high levels of clinical response in the majority of patients, though early treatment, prior to the development of TRM cells, may be the key to prolonged improved responses in patients with psoriasis.
What’s New in Targeting the IL-23 Pathway in Psoriatic Arthritis?
Professor Georg Schett
Currently, IL-23 is associated with the pathogenesis of psoriasis and inflammatory bowel disease,22 with possible involvement in PsA and spondyloarthritis (SpA).23 However, research performed in the past decade has revealed that different organs show different cytokine patterns in SpA.23 The inflammatory profile of PsA involves different upregulated cytokines; patients with joint and entheseal disease show increases in IL-23, IL-17, and IL-8, all typically elevated in psoriatic disease, as well as increases in C-reactive protein.24 IL-23 has been implicated as one of the key mediators of entheseal inflammation, activating inflammatory cytokines, and resulting in increased IL-17A, TNFα, IL-22, and polymorphonuclear neutrophil production, with localised tissue responses.25-27
In the ECLIPSA study, patients with enthesis-driven PsA receiving the standard dose of the IL-12/23 inhibitor ustekinumab showed marked improvements in enthesitis outcomes compared with TNF inhibitor treatment. Ustekinumab treatment resulted in improvements in Leeds Enthesitis Index (LEI), Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), and Spondyloarthritis Research Consortium of Canada (SPARCC) scores after 6 months of treatment.28 Suppression of subclinical enthesopathy was also clearly pronounced in patients with psoriasis during the 52-week treatment period.29
Several IL-23p19 inhibitors, which have concluded Phase III trials for psoriasis, Phase II trials for PsA, or are currently undergoing Phase III trials for PsA, are in development.30 Recent Phase II studies have shown that treatment with guselkumab 100 mg every 8 weeks (q8w) for 24 weeks results in ACR20 responses in 58% of patients with PsA, compared with 18% of patients receiving placebo (Δ40%).31 Similarly, a Phase II study showed that 43% and 48% of patients receiving risankizumab 150 mg at Weeks 0, 4, 8, and 16, and Weeks 0, 4, and 16, respectively, showed ACR20 responses at Week 24, as did 59% of patients receiving doses at Weeks 0 and 12, and 40% patients receiving a single dose of risankizumab 75 mg, compared with 31% of patients receiving placebo (Δ12%, 17%, 28%, and 9%, respectively).32 Furthermore, 73% and 71% of patients receiving tildrakizumab 20 mg or 100 mg every 2 weeks, respectively, showed ACR20 responses at Week 24, as did 77% and 80% of patients receiving higher doses of 200 mg, given either every 2 or 4 weeks, respectively, all compared with 50% of patients receiving placebo (Δ23%, 21%, 27%, and 30%, respectively).33
Speed of Response
Guselkumab treatment is also associated with a rapid onset of action in patients with PsA, as shown by the results of a Phase II study.31 Patients receiving guselkumab 100 mg q8w showed significant improvements in ACR20 scores as early as Week 4, compared with placebo (p≤0.001), which was sustained through Week 24 (p≤0.001), with approximately 60% of patients also showing sustained ACR20 responses from Weeks 24 to 56.31 Similarly, patients receiving tildrakizumab 200 mg q4w/q12w or 100 mg q12w, or were switched to 200 mg q12w, showed rapid improvements in ACR20 scores during the first 24 weeks of treatment, as did patients who were switched from placebo to tildrakizumab 200 mg q12w at Week 24 of treatment.34
Clinical Joint Response
Peripheral joint disease in PsA is characterised by several clinical factors, including IL-23 expression in PsA-related synovitis, and several peripheral joint characteristics, including a mixture of synovitis, enthesitis, and tendinitis. As such, this requires PsA-focussed clinical studies to address a polyarticular disease phenotype.35-37 The results of the DISCOVER-2 study, which examined the effect of guselkumab 100 mg q4w or q8w on ACR20 responses in biologic-naïve patients with active PsA, showed that 64.1% and 63.7% of patients achieved the primary endpoint of ACR20 response at Week 24, respectively, compared with 32.9% of patients receiving placebo (p<0.001 for both doses).38 Both doses were clinically effective at Week 4, compared with placebo (q4w: p<0.01; q8w: p<0.05), with significant differences between both doses and placebo at Week 16, the major secondary endpoint (p<0.001 for both doses).39 Guselkumab treatment also resulted in sustained effects on peripheral joint disease in the DISCOVER-2 trial, with 74.6% (q8w) and 70.6% (q4w) of patients achieving ACR20 responses at Week 52; similar responses were achieved at the same time point in patients who switched from placebo to guselkumab q4w at Week 24 (Figure 2).40
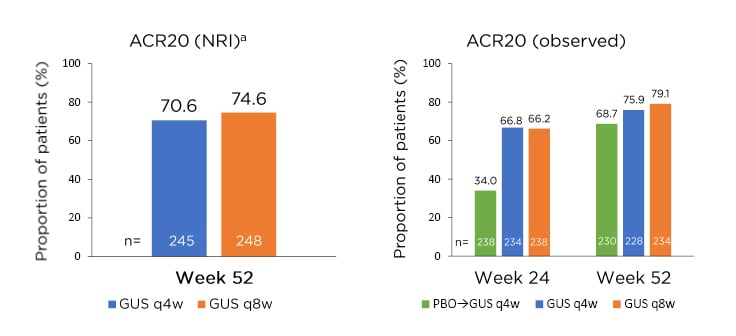
Figure 2: Sustained effect of guselkumab on peripheral joint disease in the DISCOVER-2 study with biologic-naïve patients with psoriatic arthritis.
aNRI analysis included patients randomised to q4w and q8w at Week 0 who received at least one dose of study treatment.
ACR: American College of Rheumatology; GUS: guselkumab; NRI: nonresponder imputation; PBO: placebo; q4w: every 4 weeks; q8w: every 8 weeks.
Adapted from McInnes et al.40
The results from the DISCOVER-1 trial also showed that similar proportions of both biologic-naïve and patients previously treated with TNF inhibitor achieved ACR20 responses at Week 24 of treatment with either guselkumab dose.41,42
Resolution of Entheseal Inflammation
Entheseal inflammation, another well-known characteristic of PsA, occurs most frequently in direct connection to the joints, but can also occur at sites distant to the joints.25 Erosion and enthesophyte production are also hallmarks of radiographic progression in patients with PsA.43 Patients in the DISCOVER-1 and -2 trials showed significant resolution of enthesitis (p<0.03 for both doses) and dactylitis (q8w: p<0.03; q4w: p<0.01), compared with placebo, at Week 24 of treatment.38,39 Guselkumab treatment also showed evidence of a disease-modifying effect, slowing radiographic progression, with less joint narrowing and fewer erosions, compared with placebo, at Week 24 of treatment (q8w: p=0.072; q4w: p=0.011).38,39 The results of the DISCOVER-2 study demonstrated that patients receiving either dose of guselkumab showed significant improvements in PASI scores, with 79.0% (q8w) and 78.3% (q4w) achieving PASI 75 scores at Week 24, compared with 23% of patients receiving placebo (p<0.0001).
Similar results were seen for PASI 90 and 100 scores with both guselkumab doses, compared with placebo.38,39 Both guselkumab doses also resulted in significant improvements in quality of life (QoL) and physical functions scores at Week 24 in patients with PsA.44 Prof Schett concluded by further underlining that these results solidify the role of IL-23 in immune-mediated inflammatory diseases, including PsA.
Approaches to Personalised Management of Psoriatic Arthritis: Best Practices and Optimised Care
Doctor Laura Coates
The overarching principles of optimal PsA management include concepts such as therapeutic goals; patient, clinical, and comorbidity assessments; treatment individualisation; and prompt assessments of whether these goals are being achieved (Figure 3).45
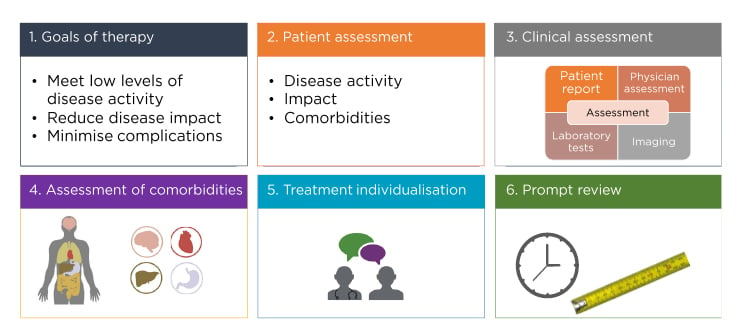
Figure 3: Overarching principles for psoriatic arthritis management.
Adapted from Coates et al.45
Several factors, including patient preferences, previous treatments, disease severity, disease domains and comorbidities, and PsA domains, play vital roles in patient-specific treatment individualisation.45 The type of PsA disease activity also plays a role, as patients may present with peripheral arthritis, skin or nail involvement, enthesitis, dactylitis, and axial disease.45
The current EULAR PsA treatment recommendations include initial treatment with nonsteroidal anti-inflammatory drugs and local glucocorticoid injections as needed, together with treatment with a conventional synthetic disease-modifying antirheumatic drug. However, these treatments are not currently indicated for enthesitis and patients with predominant axial PsA.46 Biologic treatments can include TNF, IL-17, or IL-12/23 inhibitors; should biologic failure occur, next treatment steps can include treatments with other methods of action, including JAK inhibitors (JAKi) or apremilast for patients with mild disease where biologics and JAKi treatments were inappropriate.46 Patients can be switched to alternative treatments if necessary, and cautious treatment tapering should be considered for patients showing sustained remission.46
The heterogeneous nature of PsA encompasses several disease domains depending on the type of disease activity.45
Patients with PsA often present with multiple domain involvement,47 requiring periodic re-evaluation and therapy modification during the management process.45 Previous research has also shown that patients who show the longest period of consecutive ACR20 and PASI 75 responses had the highest improvements in QoL measures such as EuroQoL-5 Dimensions Visual Analogue Scale (EQ-5D VAS) and Short Form (SF)-36 scores.48
Making the optimal treatment decision may be challenging because of the wide range of available therapies and limited head-to-head comparison data for patients with PsA. Data regarding currently available treatments, including IL-12/23, TNF, IL-17, phosphodiesterase 4, and JAKi show that all of these treatments have benefits for patients with PsA; between 40% and 70% of patients achieve ACR20 responses, and, more variably, 10% and 80% of patients achieve PASI 75 responses during treatment.39,49-53 However, studies also show that patients with axial SpA did not respond equally across treatments. For example, patients with ankylosing spondylitis receiving ustekinumab, risankizumab, and apremilast did not show any significant improvements in Assessment of SpondyloArthritis International Society (ASAS) scores, compared with placebo; however, ASAS improvements were achieved in patients receiving adalimumab or secukinumab.54-58 In the DISCOVER-1 and -2 trials, treatment with either dose of guselkumab resulted in improvement of axial symptoms, including Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores and spinal pain, compared with placebo (p<0.001), during 24 weeks of treatment in patients with active PsA and imaging-confirmed sacroiliitis.59
Diagnostic delays of 6 months or more can have a substantial impact on symptom severity in patients with PsA, resulting in increases in erosive disease, joint deformity, arthritis mutilans, sacroiliitis, functional disability, and chances of drug-free remission.60 Studies have shown that treating-to-target, with tight disease control via regular evaluations and therapy adjustments as necessary to achieve minimal disease activity (MDA), results in improved arthritis, psoriasis, and function for patients with PsA.61 Furthermore, the 2017 treat-to-target recommendations for patients with active SpA state that targets should include clinical remission and/or inactive disease of musculoskeletal and extra-articular manifestations, and that low or MDA may be an alternative target. Manifestations should also be used to define the treatment target and guide treatment decisions,62 though different remission and MDA targets may differ slightly in terms of residual disease.63
Dr Coates concluded by underscoring the importance of considering the joints, entheses, and skin when making individualised treatment plans for patients with PsA, using the simplest measures for each assessment, and using MDA or a similar endpoint as the treat-to-target goal.
Panel Discussion
The faculty responded to a variety of questions during the panel discussion. The first question focussed on the longevity of responses seen with IL-23 inhibitors, and how this applies to clinical practice. Prof Reich responded that patients participating in clinical trials have usually had psoriasis for 15 years, demonstrating the need to identify patients who might benefit from early treatment course. He also noted the need for aggressive treatment for 2 years at the onset of disease to prevent the development of chronic disease.
The online participants asked Dr Coates about the possible differences between IL-23 and IL-17 inhibitors in the treatment of axial disease. She replied that more studies should be performed to differentiate axial SpA from PsA, noting that a large project is currently planned with the ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) to improve classification criteria that can be used in future studies. Imaging studies to accurately identify and classify differences between spinal and peripheral skeletal responses are also required. When asked if skin response would be predictive of entheseal disease response in patients with PsA, Prof Schett responded that the initial data with guselkumab show that it is effective in entheseal disease resolution, similar to PASI results seen with patients with psoriasis. However, he stressed that guselkumab studies focus on a specific subgroup of patients, and that patients with increased entheseal involvement, for example, may also benefit from treatment. He noted that further studies are necessary to examine the effects of IL-23p19 inhibitors on other types of PsA.
Participants were also curious about the rapid speed of onset of IL-23 inhibitors. Prof Reich pointed out that, while IL-17 inhibitors show a fast onset of action, treatment with IL-23 inhibitors resulted in sustainable, long-term responses in patients with psoriasis. The results of the IXORA-R study show that ixekizumab results in greater changes in early treatment, compared with guselkumab,64 though speed of response is usually not the main driver of treatment choice for most of his patients. Usually, a small subset (10–15%) of patients would prefer a treatment with a rapid onset of action and would benefit from treatment with an IL-17 inhibitor. However, the majority of patients in Prof Reich’s practice, who have already had the disease for years, would prefer a treatment with sustainable, long-term responses, and would therefore benefit from treatment with an IL-23 inhibitor.
Date of preparation: June 2020
EM-33900