Abstract
Eosinophils are an important and mediate several key functions; however, they can be associated with a number of different disorders and organ dysfunction. Eosinophilia is defined as counts above 0.5–1.5×109/L, and persistent counts above 1.5×109/L are classed as hypereosinophilia. Although several systemic diseases and single-organ diseases manifest with eosinophilia, it is not always pathological. In some, eosinophilia is a key marker of disease activity, and in others, it is a reaction to another immune trigger, making it crucial to making the right diagnosis. These include helminthic and parasitic infections; immunological disorders; haemato-oncological disorders, such as hypereosinophilic syndrome; rheumatological disorders, such as eosinophilic granulomatosis with polyangiitis; and organ-specific disorders, such as asthma (respiratory) and eosinophilic oesophagitis (gastrointestinal). A systematic approach to eosinophilia is essential to make a prompt diagnosis and institute appropriate management. This article discusses the approach to a patient presenting with eosinophilia, the diagnostic work up, and management considerations. The authors particularly focus on some of the rheumatological conditions that can give rise to significant eosinophilia, and how to differentiate between these.
Key Points
1. This article discusses the key homoeostatic role of eosinophils and the pathological pathways in eosinophilic disorders.2. Included is a summary of haematological, rheumatological, and immunological causes of eosinophilia.
3. The article also discusses an approach to patients with eosinophilic disorders, in particular differentiating patients with haematological and rheumatological aetiologies.
INTRODUCTION
In clinical practice, cases presenting with eosinophilia can sometimes be challenging due to their heterogeneous nature and diversity.1 Eosinophilia can be seen across a wide range of conditions. Many of these conditions would be associated with an increased tissue eosinophil cell count and activation with the presentation based on organ dysfunction. But sometimes, the eosinophilic nature of the condition would only be demonstrated after a high clinical index of suspicion and subsequent specialised testing. Although better known for their immunological and anti-microbial role, eosinophils have critical roles in organ development and metabolism, and it is important to have a good understanding of these processes in health and under normal homeostatic conditions to understand the pathological roles of eosinophils. This narrative review was constructed in two separate parts, with the initial part focusing on eosinophils in health and disease and the second part on rheumatological diseases associated with eosinophilia. PubMed and subsequently Google Scholar searches with English language filter were performed using the terms “eosinophilia” or “hypereosinophilia” AND “rheumatology,” “arthritis,” “lupus,” and “vasculitis.” Abstracts of the PubMed results, and the title and the text of Google Scholar results, were reviewed to find any case reports, case series, or review articles, in which case eosinophilia and absolute eosinophil counts (AEC) were described in the manuscript. Additional manuscripts were identified through searches of cited articles or through personal contact.
EOSINOPHIL DEVELOPMENT AND DIFFERENTIATION
As with other blood cell lineages, eosinophil production is in the bone marrow and is led by common myeloid progenitors, which leads to an eosinophil lineage-committed progenitor (EoP).2 The EoP has a distinct surface expression phenotype and is cluster of differentiation 34 (CD34), CD38, and CD125 positive.3 These progenitor cells are seen in very small numbers in the peripheral blood and umbilical cord blood. They are more commonly seen in the bone marrow, but still only make approximately 0.05% of lineage-negative CD34+ cells. EoPs then mature into eosinophils under the influence of GATA1 gene transcription factors.4,5 These are a large family of transcription proteins that highly regulate haematopoiesis. The differentiation of eosinophils requires several factors, including high GATA1 expression and various cytokines that share common β-chain receptors such as interleukin-5 (IL-5), IL-3, and granulocyte-macrophage colony-stimulating factor.6 The most specific of these is IL-5, which is associated with selective differentiation and mobilisation of eosinophils from the bone marrow and into the various tissues, and is a very important target for pharmacotherapy in non-haemato-oncological eosinophilic disorders.7
Eosinophils released into the circulation from the bone marrow make up 1–5% of all circulating leukocytes.8 Eosinophil counts are performed by automated cell counters, which use flow cytometry to measure cell size and complexity to rapidly classify eosinophils. This can be verified on a blood film, where the cells have a distinctive appearance with granules staining bright red/pink with eosin. In the peripheral circulation, eosinophils are normally less than 0.5 x109/L.9,10 A count between 0.5–1.5 x109/L is considered as eosinophilia, and persistent counts above 1.5 x109/L as hypereosinophilia. Eosinophil migration to tissues is meditated by a family of chemokines called eotaxins. Stromal cells, including fibroblasts, smooth muscle cells, and endothelial cells, are the origin of eotaxins, although they can also be produced by epithelial cells.11,12 During inflammation, innate tissue lymphoid cells contribute to the migration of eosinophils via a complex interaction between IL-5, IL-3, and granulocyte-macrophage colony-stimulating factor.13
Physiologically, eosinophils reside in the gastrointestinal tract in much larger numbers than in the blood (about 20–30% of the total number of resident leukocytes). In contrast, certain tissues, such as the adipose tissue and the lung eosinophils, constitute much smaller proportions at ≤4% of the stromal/vascular fraction and ≤1% of total leukocytes, respectively.14 This means that the definition of excess eosinophils is dependent on the tissue and there are different values for each tissue. Typically, eosinophilic oesophagitis requires ≥15 eosinophils per high-power microscopy field (HPF) for confirmation of diagnosis.15 For eosinophilic colitis, the cut-offs are different for the different parts of the colon, with the right side harbouring much more (>50/HPF), compared to the transverse and left colon. Over 35/HPF in the transverse colon and >25/ HPF in the left colon are required for diagnosis. In the lungs, eosinophils are not seen very frequently and figures for pathological expression run at ≥2% of cells in sputum or ≥3% in bronchoalveolar lavage.14
EOSINOPHIL FUNCTION
The survival half-life of eosinophils in the peripheral blood is 3–18 hours. In tissues, such as the intestines, uterus, and lungs, they survive much longer with half-lives of approximately 6–7 days, 6 days, and 36 hours–6 days, respectively.4 Eosinophil survival can be prolonged by incubation with cytokines, and can survive for 14 days or longer in vitro.16 Eosinophils take up various roles in tissues: interacting with the various immune system components, releasing stored proteins as an immune effector cell, or participating in metabolic functions.17 Eosinophils increase the survival of plasma cells in both the bone marrow and gut epithelium through the release of survival factor activation and proliferation-induced ligand, and also facilitate the development of IgA plasma cells that play a key role in mucosal defence.18,19 Several pattern recognition receptors are expressed by eosinophils, including Toll-like receptors (TLR) 1–5, 7, and 9; nucleotide oligomerisation domains 1 and 2; Dectin-1; and receptors for advanced glycation end products. These receptors have important physiological functions in recognising pathogens and are labelled pathogen-associated molecular patterns (PAMP) and danger-associated molecular patterns (DAMP).20
Eosinophils are granulocytes, and they exert a large part of their function through the release of granules that include major basic proteins 1 and 2, eosinophil cationic protein, eosinophil peroxidase, eosinophil-derived neurotoxin, cytokines, and cytosolic Charcot-Leyden crystal protein/galectin-10.21 These granules not only have effector functions such as lysing microbe cell walls, but also activate basophils, mast cells, neutrophils, dendritic cells, and T2 immune responses.21,22 Whilst eosinophils have long been recognised to have a significant role in the immune responses against helminths and parasitic infections, it is now widely understood that they contribute to immunity against bacteria as well as viruses.1,23-26
Eosinophils are found to accumulate in the tumour microenvironment.27-29 Tumour cells can produce chemotactic factors such as eotaxin. Tumour-associated eosinophilia can be prognostically variable; for example, it has been linked to poor prognosis in Hodgkin’s lymphoma, or improved prognosis in tumours such as colorectal, breast, or prostate cancers.28,29 Eosinophils have been found to play roles in regulating fat deposition, glucose homeostasis, tissue remodelling and development, liver and muscle repair, neuronal regulation, and epithelial and microbiome regulation.30 Eosinophils also produce IL-4, and this results in the activation of muscle resident fibrocyte-adipocyte progenitors, thereby inducing regeneration of (injured) muscle and liver tissue.31 Vascular endothelial growth factor is a growth factor that can be produced by eosinophils and induces angiogenesis.
EOSINOPHIL ACTIVATION AND INFLAMMATION
In the inflamed site, eosinophils can have regulatory effects and proinflammatory and/or inhibitory effects.32 The driver for eosinophilic inflammation is T helper Type 2 (Th2) cells.33 Th2 cells produce several cytokines, including interleukin IL-5. Other cells such as Group 2 innate lymphoid cells are another potent source of IL-5 in inflammation.34 Initially described as eosinophil colony stimulating factor, IL-5 is a key factor in eosinophil maturation and activation.35 Its role includes maturing EoPs into eosinophils, promoting eosinophils from the bone marrow into the circulation, and acting alongside chemotaxins to move eosinophils into peripheral tissues, extending their lifespan and inducing activation and degranulation.36 Eosinophils have a direct effect on several different mediators. These include Type 1 cytokines (IL-12 and interferon-gamma [IFN-γ]), Type 2 cytokines (IL-4, IL-5, IL-9, IL-13, and IL-25), acute proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8), chemokines (regulated by activation, normal T cell expression and secretion, and eotaxin), and lipid mediators (platelet-activating factor and LTC4).37 Eosinophils also influence the function of several different enzymes that can lead to an anti-inflammatory effect. These include histaminase, acid phosphatase, collagenase, arylsulphatase B, phospholipase D, lysophospholipase, catalase, and nonspecific esterases.
EOSINOPHIL SUBTYPES
Two distinct eosinophil variants have been identified based on density when analysed by peripheral blood leukocyte centrifugation.38 These were classified as normodense (specific gravity >1.085 g/L) or hypodense (specific gravity <1.085 g/L). Patients with asthma demonstrated an increased number of hypodense eosinophils in the peripheral blood. Studies have suggested a correlation between this and the clinical severity of their disease.39 Inhaled corticosteroids significantly reduced hypodense eosinophils in these patients. Similar results were seen in bronchoalveolar lavage.40 More recently, eosinophils were shown to demonstrate two distinct subtypes based on other appearance features and cell markers, with IL-5 being a key differentiator. The first subgroup are resident eosinophils (rEos), which are Siglec-Fint, CD62L+, and CD101 low.14,30,41 These are IL-5 independent parenchymal cells with ring-shaped nuclei. The other subtype is inducible eosinophils (iEos), which are Siglec-Fhigh, CD62L–, and CD101 high. These cells have a segmented nucleus and are IL-5 dependent, typically found in the peribronchial area. Lung rEos were found to be in the parenchyma. In previous studies, tissue eosinophilia in patients with asthma had demonstrated to be in the peribronchial area as opposed to the parenchyma.42 Kanda et al.41 demonstrated that rEos were normodense, while iEos were hypodense. Moreover, rEos expressed genes implicated in the downregulation of the immune system, while iEos expressed several proinflammatory genes such as Slc3a2, TLR4, C3ar1, IL13ra1, and IL6. In addition to their different surface proteins, tissue location, and effect on inflammation (pro-inflammatory or anti-inflammatory), rEos and iEos were also found to differ in additional properties such as adhesion and survivability.43
SPECTRUM OF EOSINOPHILIA ASSOCIATED CONDITIONS
Eosinophilia can be the presenting feature of several different organ-based or systemic conditions. These can be dermatological, allergic, drug reactions, rheumatological, respiratory, gastrointestinal, immunological, and infections.9,44 Table 1 provides an overview of these.
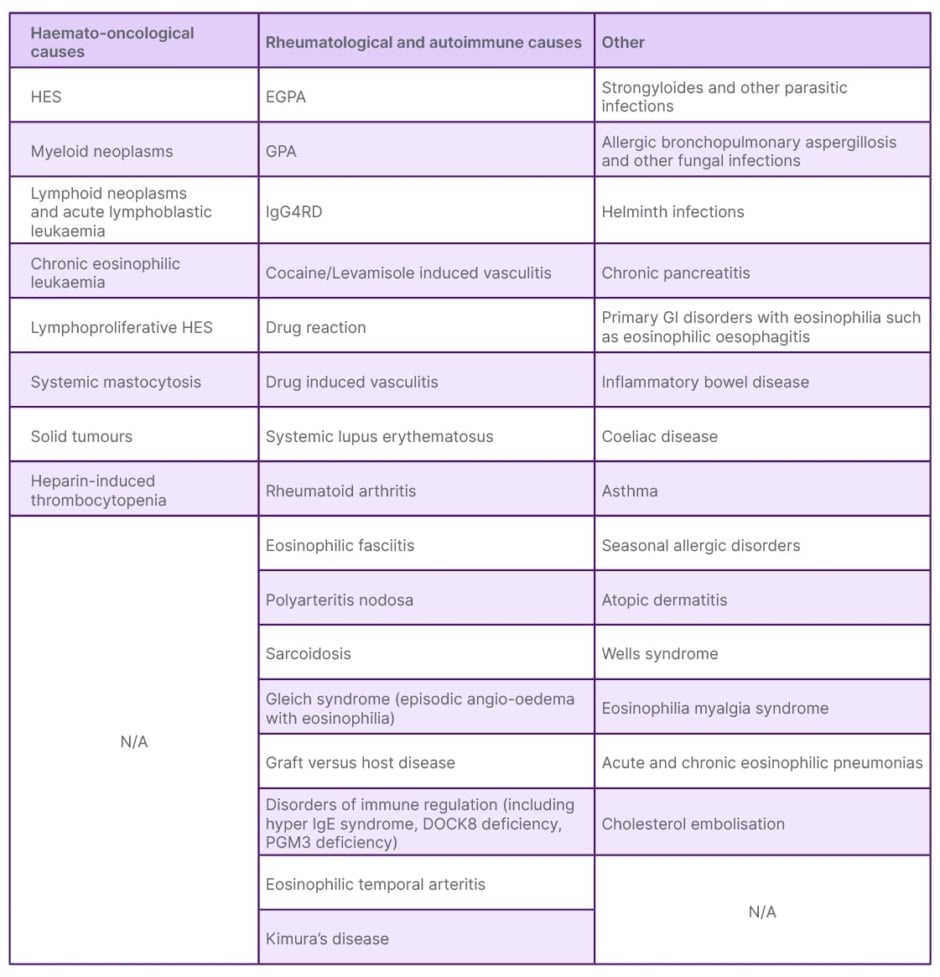
Table 1: Conditions associated with eosinophilia.
EGPA: eosinophilic granulomatosis with polyangiitis; GI: gastrointestinal; GPA: granulomatosis with polyangiitis; HES: hypereosinophilic syndrome; IgG4RD: IgG4 related disease.
EOSINOPHILS IN DISEASE: APPROACH TO EOSINOPHILIA
It is important to recognise that there can be tissue eosinophil recruitment and activation that does not result in peripheral eosinophilia. Peripheral eosinophilia can be reactive (i.e. secondary), even with very high levels, and so it is important to differentiate primary and secondary causes.45,46 Hypereosinophilia is defined as a persistent eosinophilia above 1.5 x109/L. Its definitions have evolved over time, and whilst previously a time frame of 3–6 months was necessary, this has now been amended by World Health Organization (WHO) to be simply ‘persistent’.47 Some patients with hypereosinophilic syndrome (HES) are at particular risk of organ damage, such as neurological and cardiac damage, and urgent investigations through CT or MRI head, electrocardiogram, 24-hour tape, echocardiogram, and serum troponin can be important in identifying patients requiring urgent treatment. Unprovoked venous thromboembolism should also be considered secondary to the eosinophilia. End-organ damage can occur due to any aetiology of hypereosinophilia, and treatment should be started urgently (with high-dose corticosteroids if appropriate) if there is any concern.
Helminth infections are also an important cause and would usually be accompanied by gastrointestinal symptoms such as diarrhoea, although this is not always the case.48 Although sensitivity is low, there is still value in sending stool cultures. Strongyloides stercoralis and Toxocara can often be subclinical, and serology should also be considered, given their association with eosinophilia.48 More definitive treatment is usually given only when there is a high index of suspicion for this aetiology or confirmatory evidence has been obtained.
Eosinophilia is not a common presentation in rheumatology clinics. In one study from Türkiye, high eosinophil counts above 0.5 x109/L were seen in 3.900% of patients and more than 1.5 x109/L in 0.065%.49 It is often picked up incidentally on routine blood tests or with non-specific constitutional symptoms. A detailed history including travel history, infections, allergies, and drugs is essential. History of skin rashes, lymphadenopathy, and exposure to chronic infections such as HIV are also important, alongside direct questioning for constitutional symptoms such as fever, weight loss, drenching night sweats, pruritus, and musculoskeletal symptoms. This should be supported by physical examination of the systems including the skin and joints, which are sometimes forgotten. A blood film can be helpful as automated counters may misclassify hypogranular eosinophils, and the film can highlight other features of myeloproliferative neoplasms, such as left shift, monocytosis, and basophilia, as well as circulating blasts, mast cells, or abnormal lymphoid cells.9,44,49 The degree of eosinophilia can also provide some clues to the underlying aetiology. As an example, haematological disorders such as HES can result in very high eosinophil counts (above 10 x109/L), which is not often seen with other conditions. Shortness of breath and cough are common respiratory symptoms in patients with allergic bronchopulmonary aspergillosis and this is associated with eosinophilia, and may mimic pulmonary-predominant EGPA.50 Serum testing such as for aspergillus fumigatus specific IgE and IgG, sputum testing (isolating aspergillus in sputum samples) or BAL showing aspergillus can be valuable in confirming the diagnosis of allergic bronchopulmonary aspergillosis. This, and other respiratory eosinophilic conditions can present similarly to EGPA, and differentiating them can be difficult.51,52 Several features including peripheral eosinophilia, asthma, and lung infiltrates are common to both. However, EGPA should usually involve other organs and anti-neutrophil cytoplasmic antibodies (ANCA), usually myeloperoxidase, can be positive in about 40% of cases.53 Asthma flare is often associated with a rise in eosinophil count that responds well to treatment. Gastrointestinal disorders often present with organ-specific symptoms such as reflux-type symptoms or diarrhoea and are usually diagnosed with biopsies of the relevant organs. In countries with a high prevalence of infections that can lead to eosinophilia, empirical treatment is often provided.
RHEUMATOLOGICAL CONDITIONS
Many rheumatological conditions can give rise to eosinophilia, most resulting in modest eosinophilia with counts usually less than 1.5 x109/L.49 Conditions associated with higher counts include vasculitides, drug reactions, DRESS syndrome, eosinophilic fasciitis, and IgG4 related disease. Drug reactions can cause eosinophilia that usually improves by stopping the offending drug. However, it is important to differentiate this from de novo vasculitis, which can also be caused by some drug reactions, such as cocaine-induced vasculitis.54 DRESS (drug reaction, eosinophilia and systemic symptoms) syndrome often presents with patients feeling quite unwell and often admitted to hospital.55 These patients usually have an extensive skin rash, organ involvement, fever, lymphadenopathy, lymphocytosis, and eosinophilia. Amongst the vasculitic conditions, the most common associations are for granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis (EGPA); however, there are some other less common associations such as Buerger’s disease. Eosinophilia can sometimes be seen in patients presenting as temporal arteritis, and some of these patients turn out to have EGPA. Eosinophilia can also be seen on temporal artery biopsy specimens, but there is no evidence yet that it has an impact on outcomes.56 Very rarely, EGPA and temporal arteritis can co-exist in the same patient.57 Low-grade eosinophilia can be associated with inflammatory arthritis and other rheumatological conditions such as systemic lupus erythematosus.58 Inflammatory arthritis can also be seen in GPA and EGPA, although rare and it is more common that they experience arthralgia. In very rare instances, EGPA can present similarly to rheumatoid arthritis, with destructive joint disease and nodules (with negative rheumatoid factor and cyclic citrullinated peptide antibodies); typically these nodules are eosinophilic on biopsy and associated with poor response to standard treatment.
GPA and EGPA tend to have rather different ear, nose, and throat, and lung involvement, which can help to differentiate between them. They also have different types of ANCA antibodies: the former usually has antibodies directed to proteinase 3. Patients with GPA do not usually have co-existent asthma, and nasal crusting and bleeding are the predominant ear, nose, and throat manifestations rather than nasal polyps (as is typical of EGPA). GPA is also characterised by cavitating pulmonary nodules, nasal crusting and bleeding, hearing loss, renal or other organ inflammation, and paranasal sinus erosions.59 Myeloperoxidase-ANCA antibodies, on the other hand, are typically associated with microscopic polyangiitis (MPA) and EGPA, although MPA is seldom a differential for patients with eosinophilia and does not tend to have upper respiratory tract involvement.60 Renal involvement is also quite common in MPA, whilst this is rare in EGPA. IgG4-related disease (IgG4RD) can also present with significant eosinophilia and has several different presentations. Patients with IgG4RD can have manifestations of allergy, sinus disease, eosinophilia, and pulmonary infiltrates, hence this is an important differential to consider.61 Histological features for IgG4RD include storiform, obliterative phlebitis without features of vasculitis, or eosinophilic granulomas, allowing the differentiation from EGPA. High eosinophil counts in IgG4RD may suggest a poor prognosis and can be useful in stratifying patients.62 Similarly, in patients with RA, the presence of eosinophilia has been associated with a higher likelihood of systemic symptoms and worse outcomes.63 Kimura’s disease is a rare cause of eosinophilia typically presenting with eosinophilia and raised IgE levels in East Asian populations with symptoms of lymphadenopathy, subcutaneous nodules, and salivary gland hypertrophy.64
HAEMATO-ONCOLOGICAL CONDITIONS
Hypereosinophilia can occur in several haematological disorders (Table 1), but eosinophil counts tend to be higher in HES.45 Several haematological disorders can be associated with hypereosinophilia, and once investigations have ruled these out, idiopathic HES is considered. However, HES encompasses a heterogeneous range of disorders with organ involvement, and often there is no clear underlying cause.45,65 There are several different haemato-oncological etiologies based on their pathogenetic mechanisms, and this can include a number of different malignant processes such as myeloid and lymphoid neoplasms with eosinophilia. These are associated with genetic abnormalities of platelet-derived PDGFRA (e.g. FIP1L1-PDGFRA), PDGFRB, or FGFR1. Chronic eosinophilic leukaemia or other myeloid neoplastic disease with associated eosinophilia (lacking associated genetic abnormalities) and lymphoproliferative HES-variant (aberrant lymphocytic production of eosinophil haematopoietins [IL-3/5]) is also present with eosinophilia. Idiopathic HES is typically labelled when the patient does not have any specific characteristics that would allow another diagnostic label.47,65,66 Clinically, it can be quite difficult to differentiate between idiopathic HES (when there is no cytogenetic abnormality) and EGPA, particularly given the non-specific nature of the associated symptoms, such as background of asthma, nasal polyposis, etc.67 Particularly, the ‘hypereosinophilic’ stage of EGPA with ANCA-negative status and no genetic abnormalities can be very similar to idiopathic HES. Idiopathic HES is not typically associated with nasal polyps or asthma; however, these are common co-morbidities in the general population. Idiopathic HES by definition does not have vasculitis and is typically ANCA-negative in contrast to EGPA, which requires evidence of vasculitis for accurate classification. Biopsies, however, are not always helpful in identifying granulomatous vasculitis and there remains a lack of clarity about which team should be leading the management of these patients. In fact, there do not appear to be differences in biomarkers in the two groups of patients, as evidenced by a study comparing serum biomarkers in patients with ANCA-negative EGPA and PDGFRA-negative HES.67 In fact, one patient with EGPA was found to have the genetic abnormality FIP1L1-PDGFRA fusion.52 This highlights the challenges in diagnosis and management, and this continues to remain a challenge for patients, clinicians, and academics. Eotaxin-3 has emerged as a potentially important biomarker for diagnosis, and further studies are needed to evaluate the role of this.68,69 It is important to note that joint involvement with inflammatory arthritis can occur in HES as well, but typically tends to be large joints from the author’s experience. The underlying genetic abnormalities in HES (e.g. FIP1L1-PDGFRA) cause constant activation of tyrosine kinase enzymes, suggesting that this might be a useful therapeutic target, and indeed this group of patients does respond well to imatinib (a tyrosine kinase inhibitor).70 However, response does not appear to be limited to patients with genetic abnormalities, although better response is seen in patients with these genetic rearrangements.52
CONCLUSION
Eosinophilia is associated with several different conditions some of which are rheumatological. It is important to explore the various differentials so that the right disease can be diagnosed, and appropriate management can be instituted. There are a few important rheumatological conditions to consider, and prompt diagnosis is essential as these patients can be at risk of organ damage or serious illness. The most serious rheumatological conditions causing hypereosinophilia include GPA and EGPA, whilst DRESS syndrome will also often cause significant morbidity and can be induced by rheumatological drugs such as sulfasalazine and allopurinol. In patients with hypereosinophilia that is not limited to a single organ and for which there is no clinically obvious diagnosis, genetic testing is helpful once parasitic and helminth infections have been ruled out. In some cases where there is an imminent risk of organ damage, empirical treatment is needed to ensure that hypereosinophilia is being controlled whilst the diagnostic work up continues.







