Abstract
The most common autoimmune diseases that affect the joints are osteoarthritis (OA) and rheumatoid arthritis (RA). The pathogeneses of both OA and RA are complex: in both diseases, initiation and progression are dependent on multiple joint structures, including cartilage, bone, and synovium. Mesenchymal stem cell (MSC)-based therapies are the most popular new strategy in tissue repair and regeneration, due to their multipotent differentiation abilities. In addition, MSC have therapeutic potential for bone and joint diseases through the secretion of a variety of immune modulatory substances and cell-to-cell interactions that lead to the antifibrotic, anti-apoptotic, proangiogenic, and immunosuppressive properties of the treatment. Research using MSC in various joint diseases has gained attention and impetus. A significant amount of data has shown the efficacy of MSC treatment in OA and RA, in both animal models and human trials: however, the results are often diverse and clinical benefit varies between trials. The identification of successful therapy requires further research and development, both at the basic biology and translational study levels. In this review, the authors aim to emphasise the role of MSC-based therapies in the development of treatment and to define the mechanisms involved, alongside outlining the knowledge of the therapeutic mechanisms and the applications of MSC in OA and RA.
INTRODUCTION
Joint conditions have now become a major public health problem and cause pain, functional impairment, and physical disability. Joint conditions bring patients physical discomfort and high financial expenditure. In the past, there was no effective solution to joint conditions. Patients may require surgery or face an undiagnosed condition. Until recently, cell-based regenerative therapy appeared to be a promising approach to treat joint diseases; mesenchymal stem cells (MSC) have demonstrated a positive impact on tissue repair and regeneration.1 MSC have the therapeutic potential to treat bone and joint diseases due to their multipotent differentiation abilities, the secretion of a variety of immune modulatory substances, and cell-to-cell interactions, which lead to its antifibrotic, anti-apoptotic, proangiogenic, and immunosuppressive properties.2 MSC have been tested in several clinical trials for the treatment of different joint conditions, such as osteogenesis imperfecta, osteoarthritis (OA), bone erosion, osteonecrosis, and bone fracture.3 Here, the authors review the current knowledge of the therapeutic MSC mechanisms of action and the applications of these mechanisms in the field of autoimmune arthritis.
CHARACTERISTICS AND FUNCTIONS OF MESENCHYMAL STEM CELLS
MSC were first recognised as mononuclear and plastic cells in a monolayer culture of guinea pig bone marrow by Friedenstein et al. in 1968.4 MSC are multipotent adult stem cells that are able to differentiate into mesengenic lineages such as cartilage, bone, fat, and muscle. MSC can be isolated from mesodermal tissues, such as bone marrow, synovium, cartilage, fat, and muscle, but also in endoderm (such as the thymus and the liver) and ectoderm (such as skin, dental pulp, and hair follicle) derived tissues.5-7 Currently, the majority of research looks at the therapeutic effect of bone marrow-derived MSC.
MSC are nonphagocytic and have a fibroblast-like morphology with numbers being limited in human tissues. MSC lack specific markers; therefore, MSC are still characterised on the basis of morphology, plasticity, self-renewal, and immunophenotype, which is a combination of cell markers, including CD29, CD49, CD51, CD73, CD90, CD105, STRO1, and HLA Class I-positive and CD14, CD11b, CD34, CD31, and CD45-negative. The optimal markers for selection are debated frequently: leukaemia inhibitory factor, Wnt ligands, fibroblast growth factors (FGF), other growth factors, and a variety of other cytokines, including IFN-γ, have been involved in maintenance of the MSC phenotype.8-10 MSC should meet a minimum criteria according to the International Society of Cellular Therapy (ISCT) recommendations, including surface markers CD73, CD90, and CD105-positive (≥95% expression); haematopoietic markers CD34, CD45, CD14 or CD11b, CD79a, or CD19-negative (≤2%); HLA Class II molecules absence; adhesion to plastic; and the potent of differentiation into three kinds of cells (chondrogency, osteogenic, and adipogenic phenotypes).11
MSC could improve native cell viability, proliferation, and reduce apoptosis. These effects of the MSC population are achieved through the secretion of paracrine growth factors and cytokines; direct cell–cell interactions (tunnelling nanotubes); and release of extracellular vesicles, which contain peptides, mRNA, and microRNA. MSC are able to produce soluble anti-inflammatory mediators, such as indoleamine 2, 3-dioxygenase (IDO), prostaglandin E2 (PGE2), transforming growth factor (TGF)-β, and IL-6, which mediate MSC immunomodulatory effects.12,13
MESENCHYMAL STEM CELLS AND THE IMMUNE SYSTEM
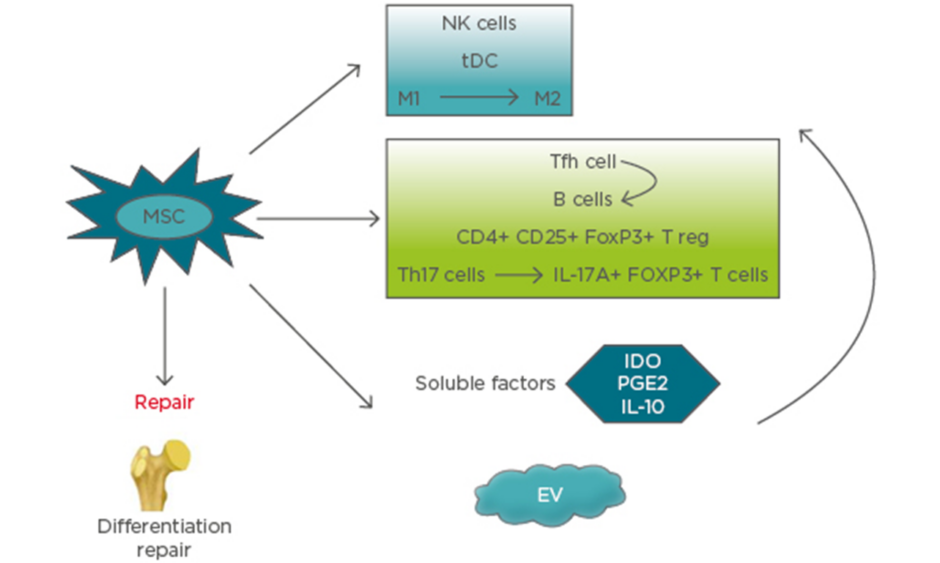
It has been reported that MSC inhibit the innate immune response. The functions of natural killer (NK) cells and neutrophils could be suppressed by MSC.14 MSC are able to interfere with dendritic cell (DC) maturation and generate tolerogenic (t)DC by inhibiting the toll-like receptor activation.14,15 The production of cytokines was inhibited by lipopolysaccharide-activated DC co-cultured with MSC, relying on paracrine mediators acting on the NFκB pathway, which was upregulated by toll-like receptor-4 induced DC activation. On the other hand, the differentiation of macrophages toward a more anti-inflammatory phenotype (M2) could be induced by MSC.16 IFN-γ secretion and the cytotoxic activities of NK cells were suppressed by MSC in vitro. In addition, suppression of NK cell function was mediated by MSC contacting with NK cells directly and via the releasing PGE2. Bone marrow (BM)-derived MSC promote IL-10-producing M2 macrophage generation that relay the cell–cell interactions and soluble mediator production, such as IDO and PGE2.17-19 MSC could also produce survival factors (e.g., IL-6, IFN-β, and granulocyte macrophage colony-stimulating factor), which increase the lifespan of leukocytes.
In addition to actions on the innate immune system, MSC also influence the adaptive immune response. Several studies have shown that the survival of T cells can be promoted by human MSC, which maintain T cells in a resting state by suppressing cell proliferation and proinflammatory cytokines production, such as IFN-γ.20 On the other hand, numerous studies have shown the ability of MSC to promote regulatory T cells polarisation as an important mechanism by which MSC inhibit autoimmune inflammation. It was also reported that MSC direct the conversion of Th17 cells into regulatory T (T reg) cells (IL-17A+, FOXP3+ T cells), which produce IL-10. In vitro co-culture of human MSC with peripheral blood mononuclear cells induces the expansion of the CD4+, CD25+, and FOXP3+ T reg population. The mechanisms by which MSC effect T cells occurs via the production of soluble mediators, such as PGE2, hepatocyte growth factor, TGF-β, IL-10, and IDO; MSC also act via cell–cell contact.21-23 All of the effects MSC produce provide a favourable environment for tissue repair. Different results were observed following MSC regulation of B cell function and proliferation. Most of the studies reported that MSC inhibited B cells proliferation, differentiation, cytokines production, and antibody production;24 however, some studies have shown that BM-MSC could promote naïve B cells proliferation, differentiation, and antibody secretion.
MSC secrete and function via extracellular vesicles (EV), including exosomes, microvesicles, and apoptotic bodies, alongside cell–cell signalling, which is a promising approach for immunomodulation therapy and tissue regeneration. The most common vesicles found in extracellular fluids are microvesicles and exosomes. The diameters of microvesicles range from 50 nm to 1 μm, which are shedding vesicles generated from plasma membranes. Exosomes are secreted by most cells as cell-derived vesicles of endocytic origin in culture systems. The mean size of exosomes ranges between 30 and 100 nm in diameter. Apoptotic bodies are released from apoptotic cells and range from 50–5,000 nm in diameter.25,26 How the effects of these MSC-derived EV are mediated is still not clear, but it represents a novel strategy for future therapy. Microvesicles have been reported to include cytoskeletal components (such as actin, actin-binding proteins, tubulin, and myosin), enzymes (such as alpha-enolase and triosephosphate isomerase), membrane molecules (such as HLA-I and HLA-II antigens), proteins involved in vesicle generation and trafficking, and proteins involved in apoptotic bodies and cell debris.25 Therefore, EV from MSC express their surface markers, such as CD29, CD73, CD19, and CD105, and cell adhesion molecules. Inside EV, there are many kinds of molecules, including enzymes, nuclear receptors, cytokines, lipid subsets, miRNAs, and other RNA (e.g., viral RNA and fragments of tRNA). It was reported that EV from MSC could inhibit the proliferation of B cells and NK cells. The effect of EV-mediated immunomodulation relies on the ability of different immune cells to take up these microparticles. The role of EV on T cells has not been clearly demonstrated. The immunosuppressive effects of BM-MSC are enhanced by IFN-γ and TNF-α, as well as an increase in the production of ICAM-1 and EV.27 EV from murine MSC were reported to inhibit the proliferation of CD8+ T cells and B cells. In addition, EV of MSC increased the T reg cell population. EV released by MSC can induce the transition and proliferation of the anti-inflammatory M2 phenotype from the proinflammatory macrophages.28,29 The mechanisms of MSC therapy for bone repair and immune system are presented in Figure 1.
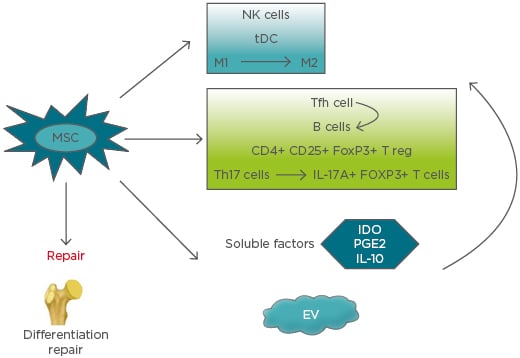
Figure 1: Mechanisms of mesenchymal stem cells therapy for bone repair and immune system.
EV: extracellular vesicles; IDO: indoleamine 2,3-dioxygenase; M1: proinflammatory macrophage; M2: anti-inflammatory macrophage; MSC: mesenchymal stem cells; NK: natural killer; PGE2: prostaglandin E2; tDC: tolerogenic dendritic cells; Tfh: follicular T helper cells; Th: T helper cell; T reg: regulatory T cells.
MESENCHYMAL STEM CELL THERAPY IN AUTOIMMUNE ARTHRITIS
Early studies have shown that MSC therapy suppressed graft versus host disease.30 Currently, most trials focus on cardiac syndromes and spinal cord injury for the reparative capabilities of MSC.31,32 In other respects, researchers studied the efficacy of MSC in autoimmune diseases due to their immunosuppressive effects. Among the autoimmune diseases causing joint conditions, OA and rheumatoid arthritis (RA) are the most common.
Mesenchymal Stem Cell for Treatment of Osteoarthritis
OA is the most common rheumatic disease, with progressive loss of normal joint function. OA is characterised by the degeneration of articular cartilage, leading to joint pain, stiffness, and loss of function. The most frequently affected joints are the knees, joints in the hands, and the spine. In addition, OA involves other joint problems, such as alterations of the meniscus, hyperosteogeny, and intermittent inflammation of synovium. Disability caused by OA is the fastest growing major health condition in the world and is expected to be the fourth greatest disease impacting on worldwide health by 2020.33
Current treatments for OA involve ameliorating pain and inflammation; physiotherapy and lifestyle regulations have been shown to slow the progression of disease, but has seen limited success.34 When conservative forms of therapy are exhausted, patients may need surgery. MSC therapy acts via two main pathways, either by preventing the degradation of cartilage through the secretion of bioactive factors, or by differentiating to become chondrocytes, which may contribute to cartilage repair. The availability of a large number of MSC and their potential of differentiating to become chondrogenic in vitro has made MSC the most promising cell source for cartilage repair. It has been reported that MSC loaded on a 3D scaffold undergo chondrogenic differentiation in appropriate culture and could be used for cartilage repair in replacement tissue.35 In an experimental model of OA, transplantation of a scaffold seeded with BM-MSC significantly improved the regenerated tissue. In addition, MSC as a cell therapy have been used directly for OA cartilage repair in the articular cavity.36 It has been shown that MSC can migrate and engraft onto multiple musculoskeletal tissues and undergo environmental specific differentiation. It was reported that little loss of proteoglycans and osteophyte formation were observed in the animals treated with MSC. Co-culture of juvenile articular chondrocytes with MSC resulted in competent chondrogenic differentiation.37-39
OA is also associated with progressive and sometimes severe synovium inflammation. Therefore, the cell therapy approach to control such an inflammatory environment is needed. MSC have been shown to regulate the immune system, so are suitable for this purpose. MSC have either direct or indirect interactions with immune cells and may secrete soluble factors that can affect their local environment. Co-culture of MSC with chondrocytes significantly downregulate several proinflammatory cytokines production. MSC could inhibit the secretion of IL-1β, IL-6, and IL-8 by chondrocytes and synovial cells, which were isolated from the joints of OA patients, relying on the secretion of PGE2 by MSC. Other research reported similar results.40-44 The expression of IL-1β, MMP-1, and MMP-13 was decreased by MSC in OA synoviocytes. In addition, a variety of factors were found in the secretome of MSC, such as TGF-β1, thrombospondin-2, insulin growth factor-1, and stromal-derived factor-1, which could increase chondrogenesis in vivo and may have a therapeutic effect for cartilage regeneration.40-44
Many of the bioactive factors secreted by MSC are now being identified, which include cystokines and growth factors, as well as extracellular matrix molecules. MSC exosomes induce high levels of IL-10 and TGF-β1, and reduce levels of IL-1β, IL-6, TNF-α, and IL-12p40 in monocytes in vitro. It has been observed that MSC exosomes induce T reg production in mice, which indicated that the MSC exosome had an immunomodulatory effect.45 On the other hand, miR-92a could mediate the effect of MSC exosomes in treating OA by targeting noggin-3 to induce proliferation of chondrocytes as well as matrix synthesis by the PI3K/AKT/mTOR pathway.46
Articular injection of MSC inhibits fibrosis and apoptosis of chondrocytes, stimulates the proliferation of chondrocytes, and increases extracellular matrix synthesis. The anti-fibrotic effect of MSC mostly relies on the secretion of hepatocyte growth factor.47 Additionally, MSC decrease the apoptotic death of chondrocytes, which were induced by camptothecin.48
In humans, several trials have started to test the efficacy of MSC therapy for treatment of OA. A case report has demonstrated cartilage and meniscus repair, identified through MRI, as well as improved range of motion and reduced visual analogue scale score after injection of autologous BM-MSC into the knee of a OA patient.49 In addition, in 18 patients with OA, the safety, function, and pain were measured at 12 months after BM-MSC transplantation. The safety is reliable, and function is improved, which continued at 30-month follow-up.50 Currently, a Phase I/II trial is evaluating the effect of MSC compared with hyaluronan in patients undergoing meniscectomy to prevent subsequent OA.51
Mesenchymal Stem Cell for Treatment of Rheumatoid Arthritis
RA is a systemic autoimmune disorder characterised by aberrant leukocyte infiltration, persistent inflammation of synovium, and proteases within the joint, which ultimately leads to cartilage and bone destruction. Treatment strategies mainly aim to suppress autoimmune synovium inflammation by using disease-modifying antirheumatic drugs (DMARD) and new therapies, such as biological agents. Even in clinical remission, cartilage damage and bone erosion may already exist or continue to progress.
MSC-induced immunomodulation and regeneration of cells made it a potential therapy for the management of RA. Therefore, unlike in the treatment of OA, the use of MSC in RA has primarily focussed on immune modulation.52 It has been shown that injection of human MSC in collagen-induced arthritis (CIA) mice decreased granulocyte macrophage colony-stimulating factor expressing CD4+ T cells in the blood and spleen, which is important in RA pathophysiology. In addition, MSC could induce a regulatory phenotype from Th17 cells by MSC in CIA mice and reduce the ratio of Th1:Th17 cells. The serum level of TNF-α was significantly decreased by MSC in CIA mice. MSC inhibited follicular Th cell differentiation in CIA mice, and suppressed their capacity of supporting B cells differentiation in a co-culture system. MSC inhibition B cells may be dependent on regulating T cells. The indirect effects of MSC on B cells may rely on suppressing follicular Th cells.53-55
MSC inhibit osteoclast-mediated bone resorption, which leads to bone erosion via the induction of T regs, and reduced the production of inflammatory cytokines, which promote osteoclastogenesis. It has been reported that MSC inhibits osteoclastogenesis via production of osteoprotegerin or by interactions with osteoclast precursors, through CD200 and CD200 receptor interactions.56 A more recent study57 suggested that injection of MSC to CIA mice prevented bone loss by decreasing osteoclast precursors in bone marrow, although the mechanisms remain unclear.
The EV produced by MSC have been shown to mediate tissue regeneration and immunomodulation as a new way to treat RA. EV secreted by MSC can mirror the effect of MSC. Studies have indicated that MSC-EV can reduce arthritis scores and pathological changes in CIA mice by decreasing plasmablast population and increasing IL-10 secretion of regulatory B cells.27
Environmental factors affect the function of MSC. Studies have shown the addition of TNF-α can reverse the immune regulatory effect of MSC on T cell proliferation. The research suggests that environmental factors, particularly those proinflammatory cytokines, may influence the immunosuppressive capabilities of MSC.58
Several studies have obtained conflicting results in clinical trials. Intravenous injection of MSC in addition to DMARD in active RA patients who had deficient responses to DMARD induced a significant improvement of clinical signs compared with the control group.59,60 In a recent multicentre, randomised, single-blind, clinical trial, intravenous injection of allogeneic MSC in active refractory RA patients showed significant clinical benefit, but these beneficial effects were not present 3 months later, which suggested that MSC treatment in RA may require repeated administration.61 Some studies, however, reported a worse outcome. Conflicting results may be caused by determinants including MSC source, origin tissue, MSC culture conditions, timing of treatment, injection cell number, and vector of injection.62 On the other hand, MSC may not be completely immune privileged. Donor-variability may also be a confounding factor. Many studies were unable to show whether these findings are reproducible using cells from different donors.63 In summary, human trials indicate that MSC are beneficial in RA, but larger, multicentre clinical studies are needed for more evidence.
CONCLUSION
In conclusion, the medical community has made advances in understanding the mechanisms of MSC therapy in joint tissue repair and their anti-inflammatory and immunosuppressive properties. Research on MSC use in various joint diseases has gained attention and impetus. A lot of data have shown efficacy of MSC treatment in OA and RA, both in animal models and human trials although the results are often diverse and clinical benefit varies between trials. Development of a successful therapy needs more effort in research and development, both at the level of basic biology and in translational studies. The authors believe that MSC therapy has great potential in alleviating the disease burden of joint diseases through their ability of tissue repair as well as the property of immune regulation.