Abstract
Biological disease-modifying antirheumatic drugs have defined a new era in rheumatoid arthritis (RA) management but share the limitation of antagonising single inflammatory cytokines or cells, as well as being either intravenously or subcutaneously administered. Following advances in the understanding of signalling pathways, the introduction of orally administered small molecules targeting key downstream intracellular factors constitutes a major breakthrough since the advent of biologics. JAK inhibition is a novel approach for treating RA and a series of agents directed against JAK have been developed for clinical use, paving the way for an innovative approach to treatment and the addition of a new class of targeted synthetic disease-modifying antirheumatic drugs to the available therapeutic armamentarium. Clinicians must now consider the place of these drugs in disease management. This review summarises the impact of JAK inhibitors and their role in the treatment algorithm of RA.
TREATMENT APPROACH IN RHEUMATOID ARTHRITIS
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disorder primarily affecting the synovial joints resulting in severe, progressive destruction of articular cartilage, subchondral bone, tendons, and ligaments. RA is the most common inflammatory arthritis, affecting 0.5–1.0% of the population worldwide.1 If not promptly and successfully treated, the condition can lead to considerable loss of function and an inability to work, having a significant impact on an individual’s quality of life and leading to an adverse social cost for the community. The last few decades have seen a dramatic change in the concept of treatment, from management strategies merely focussed on symptomatic relief and control to the adoption and implementation of a treat-to-target (T2T) approach related to the consistent measurement of disease activity in real-world clinical practice, rather than exclusively in the more formal setting of randomised clinical trials.2 Formal T2T guidelines for RA were developed several years ago,3 and similar principles based on the accurate quantification of remission or low disease activity achievement have been subsequently implemented for other rheumatic conditions.4,5 In parallel, it has also been clearly demonstrated that intensive treatment initiated soon after diagnosis is able to prevent structural damage and disease progression and improve quality of life in comparison to late treatment initiation.6-8 The benefits of early aggressive treatment of RA make up for the higher costs of medicinal products usually regarded as second-line treatment.9 This is more in line with the primary therapeutic goals set by the T2T approach of achieving remission or low disease activity, as well as having clear social benefits in terms of work impairment and quality of life.
BIOLOGICAL DISEASE-MODIFYING ANTIRHEUMATIC DRUGS FOR RHEUMATOID ARTHRITIS
In the late 1990s, the huge advances in the understanding of the cells and mediators involved in the pathogenic process of RA, specifically the role of cytokines as proinflammatory agents directly responsible for symptoms and articular damage,10 allowed for major changes in the management of the disease through the introduction of biologic agents. TNF inhibitors (TNFi) were the first biologic drugs to be licensed for RA; since then, a multitude of other single-cytokine-targeting biological agents have been approved for use. Other available biologics use a different mode of action to cytokine inhibitors, antagonising B cell function or T cell costimulation. These macromolecular proteins have markedly changed disease management and improved prognosis and outcomes in RA but, nevertheless, have also presented clinicians and budget decision-makers with challenges, with rheumatologists being at the forefront in this changing landscape.11 Although biologics can be superior to conventional synthetic disease-modifying antirheumatic drugs (csDMARD), in many situations, unresponsiveness to treatment is still an ongoing issue, and primary or secondary non-response continues to be seen in up to 40% of patients.12 Moreover, the availability of biologics has been compromised by the reality of high treatment costs. This has limited their wider adoption and restricted their use as a second-line therapy if the treatment target is not achieved with the first csDMARD strategy. The need to reduce costs has led to the successful introduction of biosimilar drugs with the expiration of patent protection for TNFi originators, and this has been greeted with enthusiasm by budget policymakers but not by all clinicians and rheumatology national societies and organisations, as highlighted by several position statements released over the last few years.13-16 Furthermore, while the high effectiveness of biologics has been described both in randomised controlled trials and real-world data, several studies over the last few years have demonstrated high discontinuation rates with biologics, with side effects and a lack of efficacy being among the main causes for treatment cessation.17 The route of administration, either intravenously or via subcutaneous self-injections, can play a role in predisposing patients to discontinue their biologics, especially in the first month of therapy,18 and implementation of regular follow-up programmes to ensure long-term adherence, in its various aspects of regularity and continuity, presents clinicians with a number of obstacles. To date, several biological agents have been licensed for use in RA, more recently followed by approval of targeted synthetic DMARD (tsDMARD), oral small molecules that block JAK, thereby inhibiting the signalling pathway.
JAK: SINGLE TARGET VERSUS A GROUP OF TARGETS
The selective inhibition of a single cytokine or cell by antagonising receptor binding on a cell surface level has not always proven satisfactory in achieving disease control in RA, perhaps because a remarkable array of multiple cytokines have been described as being important in its pathogenesis. Therefore, the logical consequence of recent advances in the understanding of downstream signalling pathways has been the development of new therapeutic agents that can provide effects across several cytokines.19 JAK are a group of four intracellular enzymes (JAK1, JAK2, JAK3, and TYK2) belonging to the larger family of tyrosine kinases. JAK proteins are constitutively bound to the cytoplasmic tail of cell surface receptors and transduce signals from a wealth of cytokines by phosphorylation of STAT factors that subsequently translocate into the nucleus, where they regulate gene expression. Multiple STAT factors have been implicated in the expression of many proinflammatory genes and are expressed in the synovial tissue of patients with RA. STAT activation correlates with disease activity in RA, demonstrating that this signalling pathway is specifically important for disease pathogenesis.20 There is increasing evidence coupling the specific JAK proteins to individual cytokine responses, although there is not yet a comprehensive and detailed description of these mechanisms (Table 1). The essential role of JAK1/3 in mediating signal transduction of IL-2, 4, 7, 9, 15, and 21 has been demonstrated, while JAK1/2 is involved in IFN-γ and IL-6 pathways. In contrast to these, the JAK2/2 homodimer has critical implications for erythropoiesis and thrombopoiesis, and mutations are notoriously associated with acute and chronic haematologic malignancies. TYK2 plays an important role in the IL-12/IL-23 pathway. Loss of their function in knockout mice has proven to lead to a phenotype of severe combined immune deficiency, defective lymphopoiesis, and erythropoiesis, supporting the potential role of JAK inhibitors as immunomodulators.21,22 In the field of kinase inhibitors, much of our knowledge is derived from oncology, based on the finding that enhanced JAK activity has been revealed in several myeloproliferative diseases. This is not the first time rheumatologists have taken and used medications from oncology. In this sense, the advent of JAK inhibitors has meant going ‘back to the future’,23 providing the opportunity to switch off a group of inflammatory pathways in RA, thus moving beyond the concept established with biologics of targeting single inflammatory cytokine or cell functions.
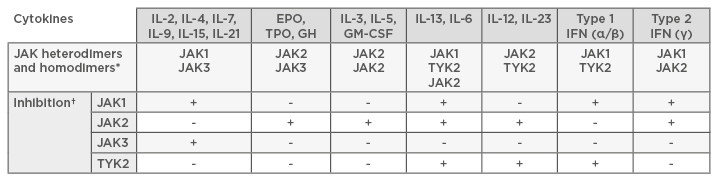
Table 1: JAK heterodimers and homodimers important for the signalling of particular cytokines.
*Different cytokines signal through different JAK combinations. †To inhibit the signalling initiated by these cytokines, particular JAK must be inhibited. This gives opportunities to design specific JAK inhibitors that reduce signaling from particular cytokines.
EPO: erythropoietin; GH: growth hormone; GM-CSF: granulocyte-macrophage colony-stimulating factor; TPO: thrombopoietin.
JAK INHIBITORS IN RHEUMATOID ARTHRITIS
The introduction of oral, small molecule JAK inhibitors (also known as jakinibs) has added a new class of tsDMARD to the available rheumatologic therapeutic armamentarium. Tofacitinib was the first JAK inhibitor to be tested in humans and was granted U.S. Food and Drug Administration (FDA) approval for the treatment of moderately-to-severely active RA in 2012. The European Medicines Agency (EMA) initially refused an application for clinical use of tofacitinib in 2013, but this tsDMARD finally received EMA approval in 2017. Tofacitinib is an oral, reversible, pan-JAK inhibitor, initially designed to be a specific inhibitor of JAK3 but then found to inhibit the kinase activity of JAK1, as well as having a small effect on JAK2 and TYK2. Tofacitinib and methotrexate in combination therapy was non-inferior to adalimumab and methotrexate in the treatment of RA in a non-inferiority, head-to-head, randomised controlled trial in patients with an inadequate response to methotrexate, without major safety concerns.24 From a clinically relevant and practical perspective, the results of this study suggested that, in patients with an inadequate response to methotrexate, the addition of tofacitinib or adalimumab was equally effective, while switching to tofacitinib monotherapy failed to achieve non-inferiority to either combination therapy. The rate of adverse events, with particular regard to those of special interest, including serious infections and malignancies, was similar between the treatment groups. Despite previous assumptions about an increased rate of herpes zoster in patients receiving tofacitinib compared to biologic-treated patients,25 the incidence was similar across all the three groups, although a possible channelling bias was acknowledged because patients at higher risk might have been more likely to receive a vaccine. A mild, but statistically significant, increase in high density lipoproteins and low density lipoproteins has also been described in clinical trials.24 Limited changes in neutrophil count, lymphocyte count, and haemoglobin levels were seen with tofacitinib treatment, but these stabilised over time in long-term extension studies, with clinically meaningful reductions in haemoglobin levels occurring in <1% of patients in all treatment groups.26
Baricitinib is an orally available, reversible JAK inhibitor with specificity for JAK1 over JAK2 and was also granted EMA approval in 2017, a few months before tofacitinib, therefore being the first JAK inhibitor approved to treat RA in the European Union (EU). The FDA was initially unable to approve the application, indicating additional data were needed to determine the most appropriate doses and to further characterise safety concerns. A resubmission to the FDA had to be filed and the manufacturer has finally announced FDA approval of the 2 mg dose of baricitinib on June 1, 2018. In a randomised, double-blind, placebo and active-controlled trial of patients who had an inadequate response to methotrexate (the RA-BEAM study),27 baricitinib was associated with significant clinical improvements compared with placebo and adalimumab. Of note, an increased American College of Rheumatology (ACR)20 score response rate at Week 12 was noted with baricitinib versus adalimumab (70% and 61%, respectively). Furthermore, baricitinib was found to be superior to adalimumab in the mean Disease Activity Score 28-joint count C reactive protein change achieved at Week 12. Rates of adverse events were similar with baricitinib and adalimumab, including serious infections. As for haematological abnormalities, baricitinib was associated with a reduction in neutrophil count, early transient increases in lymphocyte count, and modest increases in platelet count.
The pursuit of more selective therapies, particularly aiming to minimise inhibition of JAK2 and the alleged impact on haemoglobin, lymphocyte, and neutrophil counts, has focussed efforts on the development of JAK1 and JAK3 selective inhibitors. For example, filgotinib is highly specific for JAK1 and has demonstrated clinical efficacy and safety as an add-on treatment to methotrexate in patients with an insufficient response to methotrexate (DARWIN 1),28 as well as proving effective as a monotherapy, with a rapid onset of action (DARWIN 2).29
Upadacitinib, a selective inhibitor of JAK1 in development for the treatment of adult patients with moderately-to-severely active RA, has been investigated with background methotrexate in patients who had failed at least one TNFi biologic therapy (BALANCE I) and in a companion broad dose-range study comparing the efficacy and safety of upadacitinib versus placebo in patients with an inadequate response to methotrexate (BALANCE II).30,31 The safety and tolerability profiles in these Phase II studies were similar to other JAK inhibitors without obvious improved benefit-risk profiles. Results from larger Phase III trials (the robust SELECT programme) have been recently announced that showed positive results and met the primary endpoints as a monotherapy, also in patients with an inadequate response to methotrexate.32 The safety profile of upadacitinib was consistent with previously reported Phase II studies and no new safety signals were detected.
Peficitinib and decernotinib are novel selective inhibitors of JAK3 that have been shown to be effective in reducing signs and symptoms of RA and obtaining significant ACR score response rates in patients with a prior inadequate response to conventional synthetic DMARD, with limited emerging safety signals.33-35 Overall, a characteristic class safety profile is taking shape for JAK inhibitors,36 although differences among individual agents might emerge based on their selectivity. A higher risk of herpes zoster infection with most JAK inhibitors, compared to that associated with biological therapies, has been shown in real-world analysis and extension studies, thus revealing a likely class effect. However, long-term follow-up studies are necessary to assess whether JAK inhibitors are associated with an increased risk of malignancy, for instance.
JAK INHIBITORS IN THE TREATMENT ALGORITHM OF RHEUMATOID ARTHRITIS
JAK inhibitors represent a major addition to the rheumatology field and their development has expanded the number of therapeutic tools available to patients and clinicians, with a relevant impact on the treatment algorithm of RA and the guidelines endorsed by international bodies. However, recommendations vary on the optimal treatment following an inadequate response to conventional DMARD. Current European League Against Rheumatism (EULAR) guidelines for the management of RA37 recommend the addition of a biological DMARD or a tsDMARD if the treatment target is not achieved with the first csDMARD strategy and poor prognostic factors are present, although a slight preference is given to biologics over targeted synthetic drugs due to the availability of long-term safety data. This approach was also previously used in justifying the use of TNFi as the preferred first-line biologic therapy over other biological therapies due to a long-term evidence base and the availability of registry data concerning efficacy and safety.
The 2015 ACR guideline for the treatment of RA38 included tofacitinib alone as the only FDA-approved JAK inhibitor and concluded that the use of combination traditional DMARD or addition of a TNFi, a non-TNF biologic, or tofacitinib is recommended for patients with established RA with moderate or high disease activity despite DMARD monotherapy, without focussing on prognostic factors or expressing any preferences. The limited direct comparative evidence for these therapies in this clinical situation has precluded the recommendation of ranking these treatment options. In recent years, increasing interest has been shown in developing head-to-head designed studies comparing JAK inhibitors and biological products with early signs of significant differences in clinical endpoints.27 These first signals are encouraging for the use of JAK inhibitors, but it remains to be seen whether this will sanction the superiority of a mechanism of action in the long-term.
The focus on patient involvement in treatment decisions has gained a central role in current RA T2T strategies. With the advent of orally available products, this concept will need further and greater consideration in informing and updating current recommendations for selection of the optimal treatment in the setting of an inadequate response to first DMARD combination therapy. With the recent licensing for use in RA, oral targeted therapy with JAK inhibitors is now a reality, and the ease of use of an oral therapy may promote these medications to the second-line therapy of choice in the treatment algorithm of RA. In parts of the world where there is a difficulty ensuring a cold supply chain, oral therapies may also provide some advantages.
ECONOMIC CONSIDERATIONS
The economic impact of JAK inhibitors will also play a crucial role in their dissemination, as the preferential use of the least expensive therapy among treatments with similar efficacy and safety profiles is a recognised benchmark widely adopted by healthcare decision-makers at a local level. Negotiated procurement discounts for available JAK inhibitors are often provided, aiming for a cost burden close to that of biosimilars. Currently, there are limited data evaluating the expenditure of an RA treatment strategy including JAK inhibitors, although budget impact analyses, carried out in the USA where tofacitinib has been available for longer, have been encouraging, showing limited additional costs or even potential cost savings.39,40 Furthermore, with the expiration of patent protection for JAK inhibitors, we may also witness the successful introduction of medicinal products leading to even more considerable positive outcomes on budget to that achieved with biosimilars.
CONCLUSION
JAK inhibitors are novel, orally administered, effective, and rapidly acting agents for the treatment of RA. The introduction of the first non-selective JAK inhibitors constitutes a major breakthrough since the advent of biologics, overcoming the limitations of antagonising a single target through a broader magnitude of response. The oral route of administration of JAK inhibitors has the potential to minimise drug discontinuation, in contrast with parentally administered biological products. To date, international recommendations have carefully avoided expressing a definite ranking of these treatment options, but further head-to-head comparative studies may undermine this approach. The choice of the optimal treatment of active disease after inadequate response to conventional DMARD should be made by physicians through a shared decision-making process, and patients’ values and preferences could play a major role in this increasingly hot spot in the treatment algorithm of RA.








