Abstract
Background: Back pain a common cause for hospital visits. Nuclear skeletal scintigraphy, at a high sensitivity, provides a functional imaging for detecting bone diseases. Sacroiliitis is an inflammation of the sacroiliac joint. Bone scan with quantitative sacroiliac scintigraphy (QSS) has been a useful inflammation indicator for sacroiliac joints. However, QSS has been ignored in the rehabilitation practice.
Objective: To present the background, mechanisms, and current clinical applications of bone scan with QSS in spondyloarthropathy (SpA).
Methods: The authors performed a literature review of QSS through database searching of MEDLINE, Embase, CINAHL, HaPI, Cochrane Review, and citation mining. Studies were included if they had QSS in the methodology performed in adult patients with various diseases. Any articles, including the authors’, that can be performed in a clinical setting were enrolled. Articles explicitly referencing QSS were retained for screening.
Results: QSS appearance of SpA, including ankylosing spondylitis, may give rise to early detection. The specificity of sacroiliitis based on QSS increases from 73% to 97%. After investigating the relationship between serum C-reactive protein and sacroiliac joint inflammation in patients with SpA, there appeared to be a significant difference between serum C-reactive protein in serum and in sacroiliac ratio (particularly the middle part of the both joints), indicating a systemic inflammatory response to flair-up of SpA, for example, serum C-reactive protein as an indicator of inflammation. Sacroiliitis also occurs in post-streptococcal reactive arthritis. The involvement of sacroiliac joints in the development of post-streptococcal reactive arthritis had been demonstrated a significant correlation between anti-streptolysin O titres and QSS in patients with post-streptococcal reactive arthritis. Lower extremity periostitis acts as a human model in the study of bottom-up processing for periostitis-induced sacroiliac pain. The use of QSS can also monitor sacroiliac joint dysfunction before and after laser therapy. Improvements of the sacroiliac joint after convalescing of foot periostitis have been reported.
Conclusions: Bone scan using QSS is a good screening measurement in scintigraphy rehabilitation for early detection of SpA and raises awareness of physicians toward the next step of diagnosis.
INTRODUCTION
Back pain is a common cause for hospital visits. The pain origins for these patients can be determined using various methods. MRI and nuclear skeletal scintigraphy are clinical tools to determine certain aetiologies contributing to back pain. MRI is the first choice for examination over the past four to five decades. MRI produces precision images of bone and soft tissues in 3D displays. MRI is, nevertheless, not a good diagnostic tool for bone surveying and the procedure is time-consuming, costly, and has contraindications, for example the presence of cerebral aneurysm clips, cardiac pacemakers, and cochlear implants.1 Breathing movements during MRI can lead to distorted images.2 MRI produces images of specific body regions, and poor cortical bone details with only 70% sensitivity in detecting areas of active inflammation prior to the development of structural lesions.1,3
In contrast to CT or MRI, nuclear skeletal scintigraphy provides functional imaging for bones of the entire body at a reasonable cost, as well as high sensitivity for various bone diseases despite of a lower resolution of the bone scan. Such bone scans are the most widely used method to diagnose bone diseases and being critical in monitoring bone metastases.4,5 This may be posed as, in analogy, CT/MRI sees individual trees but not the forest, and nuclear medicine sees the forest but not individual trees.
Seronegative spondyloarthrithis (SpA) is closely associated with back pain and its early diagnosis is crucial. Sacroiliitis being the earlier symptom is, therefore, an important condition. Physicians, especially those of rheumatology and rehabilitation, typically diagnose SpA based on the international standards, like the Amor and Assessment of Spondyloarthritis International Society (ASAS) criteria. In the Amor criteria, 13 criteria are used to classify SpA with no image evidence.6 The diagnosis of SpA has been modified in several versions from 1990 to 2016.6-16 Before the start of SpA treatment, sacroiliitis could be caused by other diseases like psoriatic arthritis, Reiter’s syndrome, reactive arthritis, inflammatory bowel disease, or those that are inflammatory bowel disease related. These other causes need to be excluded first.17 Scientists have developed deep learning-based algorithms that can be use to detect sacroiliitis and grade the classification of SpA on plain radiograph with high sensitivity and accuracy.18,19 These approaches have overcome the interobserver variability in image interpretation. However, a group of researchers reported that technetium 99m-methyl diphosphonate (Tc-99m MDP) bone scan is more useful than plain radiographs in the early detection of SpA after study of 136 sacroiliac (SI) joints (42 patients and 26 controls) for 1 year and concluded that some patients (n=2) with negative findings from plain radiograph and MRI showed positive results in a bone scan.20
Clinicians often accentuate on the uncomfortable or tender region pointed out by the patient. Nuclear skeletal scintigraphy is an alternative method to determine the exact location of discomfort. There are four common types of scans: whole body bone scan,21 three-phase bone scan,22 single photon emission CT (single photon emission CT [SPECT]),23 and quantitative SI scintigraphy (QSS).24 These four scans all use the same radiotracer, Tc-99m MDP, which can be taken up by human skeleton. SPECT along or combined with CT as hybrid imaging can detect the exact location of neck pain in facet joint disorder,25 and raise the alarm of the sternoclavicular joint inflammation during flare-up in psoriatic arthritis.26
Although other radiotracers, such as Tc-99m-pyrophosphat, or Tc-99m hydroxymethylene diphosphonate, can be used instead, doctors of nuclear medicine still prefer Tc-99m-MDP as the choice of radiological compound.27 Tc-99m MDP is typically delivered in the bloodstream by an intravenous injection to disseminate in the body before being deposited in the bone. The primary mechanism of detecting bone lesions based on Tc-99m MDP is an abnormal accumulation due to osteogenic activity on the bone surface secondary to the calcium uptake in the affected area. Nuclear medicine bone scans can also present as a photopenic or cold zone in some bone lesions, such as the condition of bone necrosis, severe bone damage-like trauma, or tumour cell invasions leading to ischaemic changes.28,29
In this paper, the authors reviewed an alternative strategy: nuclear medicine bone scan with QSS to approach a patient with a comprehensive check-up in rehabilitation practice.
METHODS
To examine clinical application of QSS, the authors developed a search strategy of QSS using a literature review through database of MEDLINE, Embase (Ovid), CINAHL (EBSCOhost), HaPI (Ovid), Cochrane Review (Ovid), and citation mining. The research was not limited to articles published in English because abstracts in English were available. Studies were included if those had QSS in methodology performed in adult patients with various diseases. Any articles, including the authors’, that can be performed in a clinical setting were enrolled. Articles explicitly referencing QSS were retained for screening. The searches were conducted on 3rd May 2017 and updated on 31st March 2021. Summarised here are the most relevant aspects of the studies of SpA and different diagnoses of SpA according to different clinical imaging, and a critical discussion on the potential advantages and disadvantages of QSS.
RESULTS
Quantitative Sacroiliac Scintigraphy Is an Easier Way to Approach Sacroiliitis Compared to Unspecified Physical Examination
Sacroiliitis is an inflammation of the SI joint as the result of systemic diseases or stress (e.g., abnormal shearing force). The inflammation in the SI joint is also seen in individuals without SpA, including postpartum females, recreational runners, military recruits, professional ice hockey players, and healthy controls with or without symptomatic back pain.30-33 A substantial portion of those people displayed sacroiliitis and bone marrow oedema on MRI at baseline.31,32,34,35 Those structural lesions without the development of fat metaplasia have shown a more mechanical than inflammatory origin.34 In addition, the CT Syndesmophyte Score (CTSS), SI joints scores, and MRI lesions were not significantly increased in those people after a period of time, which indicated that stress may also cause sacroiliitis.31,33,34,36
Clinical manifestations of sacroiliitis are lower back pain, inguinal ligament pain, or buttocks pain, and even radiated pain to hamstrings similar to sciatica.37
The Fortin finger test may indicate the area potentially generating the pain by asking patient to point at the area of pain to the clinician.38 Those patients who fail to point out their painful areas would be identified to have poor outcome of their disease. Patrick’s test is another method of physical examination for sacroiliitis. In this method, the examiner stretches the patient’s SI joints according to the following motions: flexion, abduction, and external rotation of the hip joint.39 A positive test result is one in which refers to back pain or buttock pain, whereas groin pain is a sign that is more indicative of sacroiliitis.39 In addition, Gaenslen’s sign is a test of sacroiliitis by abducting one side of hip joint and extending the other side in counter-rotation.40 The result is same as in the Patrick’s test.40 Another test for sacroiliitis is the posterior superior iliac spines (PSIS) distraction test.41 A positive result is feeling pain when a medial-to-lateral direction force is applied on PSIS.41 In brief, there are many methods to test for sacroiliitis, but all with low specificity.42
Nuclear medicine bone scan with QSS has been a useful inflammation indicator for SI joint for the last 50 years. QSS is typically done following an intravenous injection of about 750 MBq Tc-99m MDP. Planar imaging of the spine and SI joints achieves in the antero-posterior projections 3–4 hours after the injection, using a gamma camera via an elliptical course (360°, 64 projections, 20 sec/projection) the camera acquires images around the SI joints, storing in 128×128 matrices. Using the region-of-interest (ROI) method, a quantitative SI joint-to-sacrum ratio (or SI ratio), is computed based on counts at similar regions measured at the SI joint and at the sacrum.43 The ratio for the upper, middle, and lower parts of both joints is measured individually (Figure 1). In brief, the inflammation is considered as negative if the ratio is <1.3, or equivocal if around 1.4, or positive if >1.5.
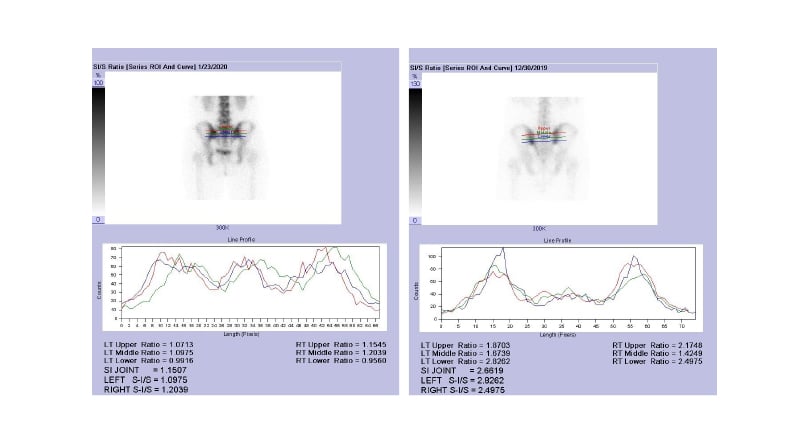
Figure 1: Methods used to obtain a quantitative sacroiliac joint-to-sacrum ratio.
From the ROI based on accountable images, the ratio is obtained after measuring total number of counts within the region of SI joint and divided by total number of counts within an equal-size region at the sacrum. The ratio for the upper (red), middle (green), and lower (blue) parts of both joints can be measured individually.
Left panel: a subject with normal SI joint. Right panel: a patient with AS.
AS: ankylosing spondylitis; ROI: region of interest; SI/S: quantitative sacroiliac joint-to-sacrum ratio.
In 1977, Buell et al.44 were the first to report higher SI ratios in patients with SI disease. In 1998, Kaçar et al.45 reported that for subjects 20 to 60 years old, the normal ranges of counts of SI joint versus over counts of sacrum ROI were between 0.74 and 1.22 for females, and between 0.87 and 1.31 for males. The reference SI ratios were estimated to be an average of <1.3200 for normal subjects and <1.3812 for late arthritic patients, while even higher at 1.5200–2.0900 for those with early arthritis. It was reported that patients with radiographic Grade I–II were considered to have early arthritis and patients with radiographic Grade III–IV were considered to have late arthritis.45 Tiwari et al.46 later showed four quantification methods of the SI joint index: irregular ROI, rectangular ROI, profile peak counts, and profile-integrated counts. All of these methods gave similar results. Sebastjanowicz et al.47 reported a range of SI ratio between 1.18 and 2.28 for control subjects, with the highest standard deviation in paediatric patients. Therefore, SI ratio is a good measure for detecting early but not late arthritis.45 The maximum and minimum of SI values need to be considered for younger (<20 years old) and older patients (>61 years old).47 SI ratios can be measured individually as the upper, middle, and lower third due to distinct anatomic structures and kinetic physiology in the three parts of the joint on the bilateral sides.48
Unlike adults,49,50 children’s quantitative SI indices may give good results using L5 as the background51 or with the use of MRI because these younger subjects have more synovial enhancement without bone marrow oedema.52
The earlier study regarding QSS in healthy people was performed in a medical centre where a posterior planar film of the pelvis was obtained 3 hours after injection of 740 MBq 99mTc-MDP and the SI ratio was calculated.53 The results showed that the age-related changes in SI ratio are significant between sexes in certain age groups, but not lateralisation, and the SI ratios dropped steadily with age in females, whereas two plateaus appeared at ages 21 to 40, and 41 to 70 years in males.53
DISCUSSION
Quantitative Sacroiliac Scintigraphy for Spondyloarthropathy, Including Ankylosing Spondylitis
Ankylosing spondylitis (AS) is a disease of axial SpA but is often recorded as suspicious by radiologists after a plain X-ray, and is, therefore, sometimes diagnosed late. In the 1980s, SI ratio was being used as important indicator in early vigilance of AS, particularly in males to prevent its progression to sacroiliitis.49,54-57 Although QSS showed a limited sensitivity and specificity of approximately 50% for each when appropriate controls were used,58,59 the SI ratio of 1.55 meant that AS disease progression was expected to last <3 years and >3 years at 1.40.60
In 1993, Collie et al.61 measured SI ratios for six of 11 patients (5 female, 6 male), and found elevated ratios (>1.5 for both SI joints) in four of them. After 2 years, two of these patients showed higher SI ratios that preceded plain roentgenologic abnormalities. In two patients, unilateral sacroiliitis on plain radiographs was confirmed as bilateral on QSS. They suggested that the scintigraphic appearance of AS, though not unique to the disease, offers the opportunity for early detection and vigilance, avoiding further unnecessary investigation and treatment for both suspected as well as unsuspecting cases.61
In addition, Koç et al.62 reported that with SI ratios, the specificity of sacroiliitis based on bone scans increases from 73% to 97% and negative predictions increase up to 91%, in parallel with positive predictions. The authors of this current study concluded that with regard to time and cost, bone scan is slightly better than MRI and SPECT/CT in detecting AS and sacroiliitis.62
Elevation serum levels of high sensitivity C-reactive protein (hs-CRP) are considered a risk factor/biomarker for various diseases, including SpA. A previous study had retrospectively investigated the relationship between serum hs-CRP and SI joint inflammation in 29 patients with SpA (n=29; mean age: 32.27 years; female–male ratio: 6:23). All patients underwent hs-CRP testing and skeletal scintigraphic scans with QSS between January 2007 and September 2013. The results showed a significant difference between hs-CRP in serum and in SI ratio (particularly the middle part of SI joint, on both sides). It was concluded that the significantly high concentrations of serum hs-CRP may indicate a systemic inflammatory response to a flair-up of the SI joint and should be an indicator of SI inflammation in SpA.63
Although QSS may not be mandatory for most patients with suspected AS, an empirical practice has depicted that it has a role in selected cases where there is a very visible disorder in the absence of obvious roentgenologic changes. For accurate diagnoses of SpA, QSS is only recommended when combined with a CT scan.64
The importance of concomitant use of CT in the assessment of SI joints along with scintigraphy includes the following: the combination of semi-quantitative analysis of CT and quantitative analysis of SI joints can increase the unique specification of the risk level for active sacroiliitis;65 CT is the gold standard for bone erosion and superior to conventional radiography and MRI,65 it enables the cross-sectional, multi-planar visualisation of the pathologic processes, which was better than conventional radiography,66-68 in addition, the Modified New York Criteria scoring system for sacroiliitis can also applied to CT; by using spectral CT, fat deposition and bone marrow oedema can be measured similarly to MRI, which can increase the sensitivity for early changes of sacroiliitis;65,68 and, other than bony erosion and relative water and calcium ratio of the SI joint, CT can detect sclerosis and syndesmophytes, which could be helpful to identify differential diagnoses in chronic changes in sacroiliitis.65,68
Quantitative Sacroiliac Scintigraphy for Post-Streptococcal Reactive Arthritis
Titers of anti-streptolysin O (ASLO) are of diagnostic value in the early detection of post-streptococcal reactive arthritis (PSRA),48,69-71 early arthritis after rheumatic fever,72-74 and movement disorder.75 The dividing line is normal if the titer is ≤116 IU/mL, and abnormal if the titer is >116 IU/mL. PSRA is a non-suppurative sequela of a prior streptococcal infection.
In Asia, some scholars have demonstrated the involvement of the SI joint in the development of PSRA. In a study, a total of 84 subjects (mean age: 23.0; range: 18.0–36.4) underwent QSS and their ASLO titers were measured (range: 25–520 IU/mL). The SI joint was divided into three regions: upper, middle, and lower parts. Depending on fibrous cartilage, ligament, and the direction of the SI joint, bilateral QSS measurements of the three parts were collected. A highly significant correlation was found between ASLO titers and SI ratios (p<0.0001). An increment of 1 IU/mL of the titer resulted in a significant increment of SI ratio by 0.0008 units. It was also found that with the increased ASLO titer per unit, SI ratio increased significantly by 0.0008 units and thereafter 0.0074 units per additional year.48 The findings suggested that SI joint involvement is a manifestation of PSRA. The results demonstrated a strong correlation between the ASLO titer and the QSS in patients with PSRA. Subjects with SI joint involvement should be advised to have an ASLO titer measured and a QSS done.48
It was noticed that those patient with SI also reported upright postural abnormality. It was, therefore hypothesised that an imbalance of the lumbopelvis due to SI disorder might produce a positional change, and there would be different postural sway when standing upright. All subjects underwent 10 sway tests to assess static sway in an upright standing posture. With eyes open and plantar flexion, the high ASLO group had bigger values in sway area, sway velocity, and sway intensity. The values of sway velocity and intensity obtained with eyes open and plantar flexion and dorsiflexion had lower intensity values when compared with those obtained in closed eyes and plantar flexion and dorsiflexion in the high ASLO group, but not in the low ASLO group. Significant differences were found between the two groups in all sway values under all the tested position conditions. It is speculated that subjects with high levels of streptococcal serology have greater sways on all postural parameters compared to those with low serology. The speculation is consistent with proprioceptive deficits in the SI joint contributing to postural impairments,76,77 as shown in Figure 2. The use of QSS is the first of its kind to detect active SI joint disorder in the studies of PSRA.
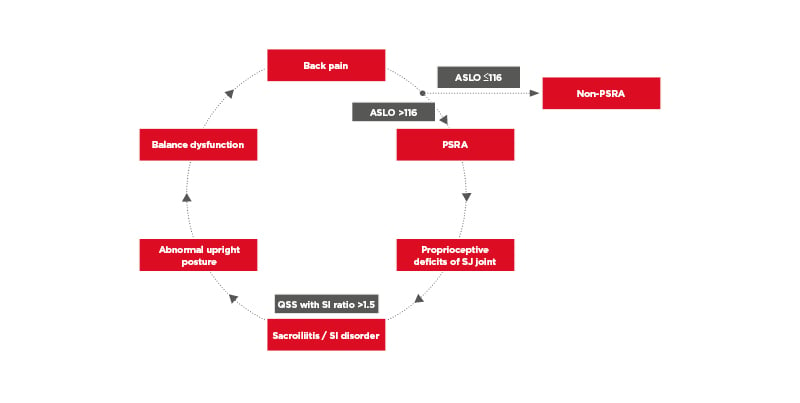
Figure 2: Schematic illustration of the pathophysiology of post-streptococcal reactive arthritis and its related disorders.
ASLO: anti-streptolysin O; PSRA: post-streptococcal reactive arthritis; QSS: quantitative sacroiliac scintigraphy; SI: sacroiliac.
Quantitative Sacroiliac Scintigraphy for Osteitis Condensans Ilii
Osteitis condensans ilii (OCI), first reported in 1926, has symptoms that include axial lower back pain and premature arthritis.
It primarily affects females younger than the age of 40, and often after pregnancy. Parperis K et al. have suggested the term idiopathic pelvis sclerosis might be an alternative term to OCI due to the lack of inflammation evidence in those patients.78 OCI is considered a diagnosis of exclusion because plain radiograph and MRI findings of the spine were unremarkable;79 while the characteristic X-ray image may show triangular sclerotic changes in the auricular portion of the bilateral ilium with preserved SI joint space.80,81 Patients mostly exhibit normal physical and neurological examination but some show SI joint region tenderness and increased lumbar lordosis.59 From a case-control study, OCI is shown to be associated with pain on SI stress tests. The preserved SI joint space is the key characteristic of OCI.79 It has been postulated that OCI induces stress on the SI joint, leading to a piriformis muscle syndrome with sciatica. The association between OCI and SI joint stress, and that between sacroiliitis and sciatica, has been reported. It is characterised by a favourable prognosis;78 therefore, the treatment goal was to improve their health-related quality of life.78 In clinical practice, symptoms of OCI presented with piriformis muscle syndrome and sciatica could be successfully managed with SI joint injections.82 The use of QSS is the first of its kind to monitor active SI joint disorder before and after the treatment of OCI. MRI, SPECT, and PET/CT might have the ability to distinguish OCI from other diseases and require larger-scale studies before use in the clinical setting.81,83,84
Quantitative Sacroiliac Scintigraphy for Periostitis-Induced Sacroiliac Pain
Juvenile-onset HLA-B27-associated ‘unclassifiable’ SpA, especially cases without evidence of enteric or genitourinary symptoms, may have tarsal periostitis as an early clinical manifestation and then show bilateral sacroiliac pain later on.85 One article has reported improvements of the SI joint after convalesce of the foot periostitis, and concluded that stress events, exercise, and abnormal posture can all increase SI ratios, while corrected posture for a period of time, anti-inflammatory drugs, and rehabilitation programmes can all reduce SI ratios.86
Periostitis of the lower limbs is a common disorder from sports injuries. Foot periostitis is considered a human model in the study of bottom-up processing. Calin et al.87 have demonstrated that pelvic radiography showing fluffy periostitis was equally distributed among symptomatic, asymptomatic HLA-B27-positive, and symptomatic HLA-B27-negative control groups.87 The diagnosis of foot periostitis, therefore, can be confirmed by medical history and physical examination, as well as triple-phase bone scan using skeletal nuclear scintigraphy. One clinical study88,89 (n=54) explored functional improvements of the lower limbs after low-level laser therapy (LLLT) with regard to balance function, including postural stability testing and limits of stability. After therapy, there were significant improvements in pain score and balance dysfunction. The study concluded that LLLT is effective for treating lower-limb periostitis and even in short-duration interventions, and LLLT exerted a positive effect on proprioception in these patients.88,89
Furthermore, foot pain was hypothesised to induce defective biomechanics and might cause SI joint stress and convalesce of the foot periostitis could restore the abnormal SI joints. The results showed scintigraphic improvements in the SI ratio, indicating significant therapeutic effects on foot periostitis. There was also a significant association between the middle and lower parts of the SI joint. SI ratios for the middle part on both sides were significantly higher (0.06 units) compared to the lower part. In conclusion, the patients with SI joint stress, as the result of bottom-up processing of foot periostitis, could be treated successfully after convalesce of the foot periostitis by either LLLT or conventional treatments.86 In another report regarding medial tibial stress syndrome (MTSS), similar findings were also reported. Both MTSS and SI joint stress can be confirmed by nuclear scintigraphy.90
Outcome measures included the Lower Extremity Functional Scale (LEFS) and QSS. The results showed that after therapy, LEFS was significantly higher (38.45, p<0.0001), and QSS was significantly lower (p<0.0001). There is also a significant association between the middle and lower parts of the SI joint. It has been confirmed that SI joint stress due to bottom-up processing of MTSS can be normalised after successful therapy of MTSS by LLLT.90 The use of QSS is the first of its kind to monitor SI joint dysfunction before and after the studies of periostitis-induced sacroiliac pain.
Limitations of Quantitative Sacroiliac Scintigraphy
QSS has several limitations. The first limitation is lower specificity in detecting chronic inflammation. SI values the in acute phase of stress-induced inflammation fall between 1.7 and 1.8 but nearly normal values tend to be reached after 6 months of recovery. The hyper-fixation method for the SI joints may be used in treating chronic SI arthritis. The second limitation is related to the small overlaps in SI indices between lower back pain and sacroiliitis patients and controls.91 Therefore, the reference values and the cut-off values of SI joints need to be clearly established.38 In addition to this, stratification of age27,53,91 and sex45,53 are needed to distinguish the overlapping area resulted from sacroiliitis. One literature review has included studies about well-defined AS populations only, suspected sacroiliitis, and/or mechanical lower back pain.92 They excluded the studies without preforming the SI joint–sacrum ratio or giving a clear definition of reference value of the SI joint–sacrum ratio in quantitative scintigraphy and calculating sensitivity or specificity value.92 They have concluded that although scintigraphy was nearly 50% cheaper and generally more available than MRI, the likelihood ratio of scintigraphy for diagnostic acute sacroiliitis was only between 2.5 and 3.0.92
The authors of the review suggested that QSS was limited for diagnostic values for AS diagnosis.92 Using auxiliary laboratory data and clinical scoring, including serum hs-CRP, vitamin D levels, erythrocyte sedimentation rate, and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), clinicians may able to detect SI inflammation-related diseases from healthy controls.93 A current article has suggested there is value of QSS in helping the differential diagnoses of back pain-associated conditions, such as suspected fracture,64 particularly in survivors with SI joint pain after experiencing a vehicle accident.
CONCLUSION
In this article, the authors have revisited skeletal scintigraphy using QSS on patients with SI joint dysfunction, who also present with myalgia, arthritic, or lower back pain. More than one underlying disorder may contribute to symptoms in these patients. Clinicians need to better understand the pathophysiological mechanisms and the origin of sacroiliitis before prescribing treatments. QSS is helpful in clarifying inflammation of SpA and in disease assessment. In conclusion, the skillful pattern recognition skills of the diagnosis of axial SpA are more important than overdiagnoses by a positive MRI finding. Bone scan using QSS is a good screening and follow-up measurement in scintigraphy rehabilitation for early detection and vigilance of SpA and raises awareness for physicians to adopt the SI ratio in the next steps towards diagnosis.







