BACKGROUND AND AIMS
Lenabasum is an oral, non-immunosuppressive, cannabinoid Type 2 receptor agonist that activates resolution of innate immune responses.1 In animal models of bleomycin-induced skin and lung fibrosis, lenabasum reduced inflammation and fibrosis.2–4 In a Phase II study in diffuse cutaneous systemic sclerosis (dcSSc), lenabasum was safe and well-tolerated, and was associated with greater improvement in the American College of Rheumatology composite response index in systemic sclerosis (ACR CRISS) score at Week 16 than treatment with placebo.5
RESOLVE-1 was a Phase III study to further test efficacy and safety of lenabasum in adults with dcSSc ≥6 years duration. Background immunosuppressive therapies (bIST) were allowed if doses were stable for ≥8 weeks before screening, to reflect current standard-of-care practice of treating early dcSSc with IST. The primary efficacy end-point was the ACR CRISS score at Week 52,6 comparing lenabasum 20 mg and placebo groups.
MATERIALS AND METHODS
From 76 sites in North America, Europe, and Asia, 375 subjects were randomised 1:1:1 to lenabasum 20 mg, 5 mg, or placebo, all twice daily. Subjects in the three groups had similar demographic and baseline disease characteristics, with moderate to severe disease overall. Mean disease duration was approximately 32 months, and modified Rodnan skin score was approximately 22 in each group. Subjects were heavily treated with bIST: 84% on any bIST; 51% on monotherapy bIST; 33% on combination bIST; and 51% on mycophenolate mofetil (MMF).
RESULTS
The ACR CRISS score at Week 52 was not significantly different between lenabasum 20 mg and placebo groups (Table 1).
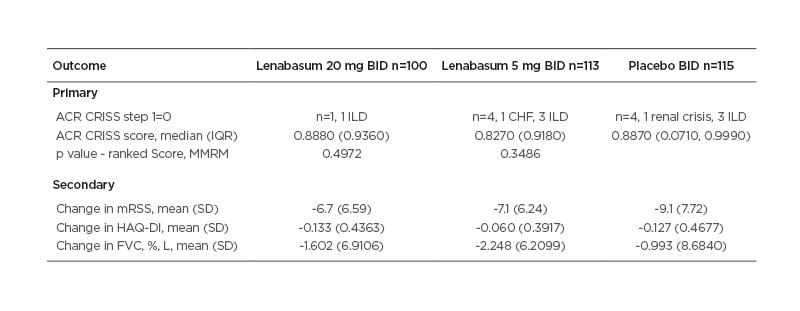
Table 1: Primary and secondary efficacy outcomes.
Improvement in the placebo group far exceeded expectations based on literature and expert opinions, and the authors were unable to discern treatment effect from the placebo effect.
mITT population: missing visits or the ACR CRISS score core items due to COVID-19 were imputed using LOCF. For other missing data for any core items, imputer used MCMC multiple imputation technique prior to calculating the score, but missing visits were not imputed.
Combined inference statistics: each imputation was analysed by MMRM on the ranked ACR CRISS score with region, disease duration, baseline mycophenolate use, visit, treatment, and treatment-by-visit interaction as the fixed effects and baseline mRSS as a covariate. Secondary outcomes were similarly analysed but using MMRM without a ranked score.
ACR CRISS: the American College of Rheumatology composite response index in systemic sclerosis; BID: twice daily; CHF: congestive heart failure; FVC: forced vital capacity; HAQ-DI: health assessment questionnaire-disability index; ILD: interstitial lung disease; IQR: interquartile range; LOCF: last observation carried forward; MCMC: Markov chain Monte Carlo; mITT: modified intent-to-treat; MMRM: mixed model repeated measures; SD: standard deviation.
Indeed, all three groups had median ACR CRISS scores >0.82, likely speaking to the efficacy of bIST and the ceiling effect of ACR CRISS score. Secondary outcomes showed no significant advantage to treatment with lenabasum. Modified Rodnan skin score improved in all three groups, with mean improvements of 7–9 points at Week 52.
In pre-specified analyses, MMF was found to have a significant effect on ACR CRISS score and certain secondary efficacy end-points, including change in forced vital capacity (FVC). In post hoc analyses, effects of MMF on the ACR CRISS score and change in FVC were more pronounced when treatment duration was shorter at study entry. In other post hoc analyses, subjects on established bIST (≥2 years treatment duration) who received lenabasum 20 mg had stable FVC, whereas those treated with placebo experienced a decline, a difference that was nominally statistically significant.
Lenabasum-treated subjects had numerically fewer serious and severe adverse events than subjects treated with placebo. Adverse events that were more common in lenabasum-treated subjects included dizziness, headache, dry mouth, and somnolence, none of which were serious. Neutropenia, opportunistic infections, and malignancies occurred with similar frequencies in lenabasum and placebo-treated groups, consistent with lenabasum not being immunosuppressive.
CONCLUSION
In conclusion, this study did not demonstrate efficacy for lenabasum in subjects with dcSSc who were receiving standard treatments including substantial immunosuppressive therapies. Of note, the ACR CRISS scores in all groups far exceeded what was expected, suggesting a possible ceiling effect of that outcome measure in trials that allow bIST. Mycophenolate treatment significantly affected the results, with greater improvement seen in subjects who received MMF. That effect was less apparent in those whose treatment duration with MMF was >2 years at baseline. The suggestion that lenabasum may afford less decline in patients on stable bIST will require confirmation and additional studies.








