Meeting Summary
The third Encuentro Latinoamericano de Infecciones Respiratorias Recurrentes (ELAIR) took place in Mexico City, Mexico, on 11th–12th May 2017. ELAIR brought together experts from across Latin America and further afield, continuing an extraordinary didactic exercise on the cutting-edge advances of respiratory medicine. Impressive progress has been made in the past 15 years, with new treatments available to manage and prevent airway infections. It remains to be seen how this might affect the related conditions of wheezing and asthma in predisposed and sensitised subjects. However, early data suggest that lower respiratory infection rates may reduce the development of the above conditions which are closely related to viral infections. Immunomodulators that both prime the immune system to fight infection and reduce inflammation are likely to play a major role in secondary and even potentially primary prevention of atopic diseases.
CHRONIC AND RECURRENT RESPIRATORY TRACT INFECTIONS IN ADULTS: DISEASE BURDEN
Upper respiratory tract infections (URTI) have a significant societal impact as leading causes of physician visits and work absenteeism in adults. In developed countries, acute respiratory tract infections (RTI) account for 20% of all medical consultations and 75% of all antibiotic prescriptions.1 The economic burden of RTI is considerable, with figures from the year 2002 suggesting that >$2 billion was spent on over-the-counter medications in the USA, and the annual cost of acute URTI to the UK NHS was estimated at £60 million.2
Respiratory tract pathologies have the same names in childhood and adulthood, yet the presentation in the context of the developing versus fully developed respiratory and immune systems may be substantially different. Chronic rhinosinusitis (CRS) is a frequently encountered condition in adults and is defined as a persistence of sinus inflammation for >3 months. Symptoms include nasal obstruction, congestion, rhinorrhoea, anterior discharge, posterior drip, and facial pain. Secondary to these symptoms are headache, facial pressure, ear pain, halitosis, cough, dental pain, fever, and fatigue. Given their persistence, the impact on the quality of life (QoL) of a sufferer may be significant.
CRS affects approximately 11% of the population throughout Europe and 14% of the population in the USA.3-6 Individuals with both acute disease and CRS consistently score below the general population in both physical and emotional aspects related to QoL, as assessed by the 36-Item Short Form Survey (SF-36) (Figure 1).7,8 In light of the societal and personal impact, numerous evidence-based guidelines have been published to raise awareness within the clinical community and improve the diagnosis and management of rhinosinusitis and its subtypes.3,4
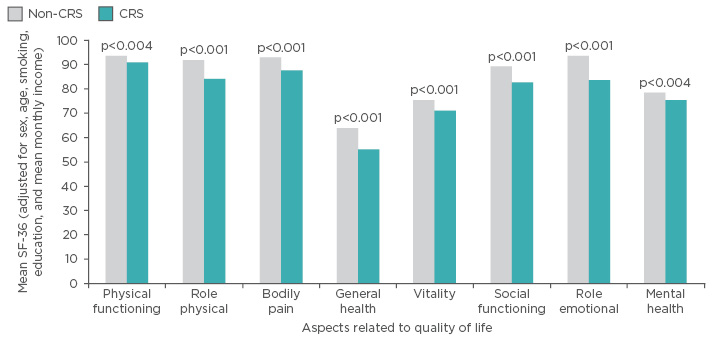
Figure 1: Impact of chronic rhinosinusitis on quality of life in a large Chinese population-based survey (N=1,411).
CRS: chronic rhinosinusitis; SF-36: 36-Item Short Form Survey.
Adapted from Fu et al.7
Those adults at a higher risk of URTI are collectively known as ‘fragile adults’. Typical factors increasing the risk of URTI include an impaired immune response, which may be due to immune deficiency or other immunity-related issues, such as atopy. Environmental and behavioural risk factors include smoking and exposure to pollution.9 Chronic respiratory disease also predisposes patients to acute infection due to physiological alterations.10,11 In the elderly, impaired phagocytosis and the process of immunosenescence are known to increase this risk.11
The vast majority (90%) of RTI are caused by viruses. Misuse and overuse of antibiotics in the treatment of RTI, particularly in CRS, contribute to antimicrobial resistance. Antibiotics can also cause collateral damage by impairing the host microbiota, which creates a substrate for further infections. Beyond the impact on those individuals directly affected, antimicrobial resistance in respiratory disease also represents a risk for dissemination of resistant infection to the wider population. As a result, prophylactic antibiotic use for RTI is only appropriate in exceptional circumstances and decreased use of antibiotics is an urgent priority.12-17
Effective management of URTI is a priority due to the significant individual and broader socioeconomic impact.18,19 The majority of treatment remains focussed on symptom relief using antipyretic and anti-inflammatory drugs because, in most cases, infections are viral and not amenable to antimicrobial therapy. Given these limited options, the focus of management should be weighted towards prevention rather than treatment. Behavioural interventions and targeted surgical treatment may be of use in adults, particularly fragile adults. The use of immunomodulators to bolster the defensive capacity of the immune system offers an additional prophylactic strategy available to adults suffering chronic URTI.20
RECURRENT RESPIRATORY TRACT INFECTIONS IN CHILDHOOD
Recurrent respiratory tract infections (RRTI) are very frequent in childhood and have the potential to be extremely severe. From the immunological perspective, children face a hostile world from the moment of birth, with threats represented in the form of antigens. Parental defence against this hostile environment extends to the immune system; however, at 6 months of age the passive immunity conferred by maternal immunoglobulins (Ig) expires, and immunological immaturity confers an environment where RRTI may exceed normality in terms of frequency and severity.
After reaching a nadir at approximately 6 months of age, when maternal IgG can no longer be absorbed due to gut closure, IgG levels steadily increase, reaching approximately 80% of adult levels by 5 years of age (Figure 2). However, the principal Ig for protection against respiratory immunity, IgA, only attains 40% of its adult levels by 5 years of age (Figure 2). As a result, immune immaturity is particularly marked in the respiratory system. Maturation of all aspects of the adaptive immune system is modulated by the innate immune system; this system also shows functional immaturity after birth and, in some infants, abnormalities in innate immunity will have downstream effects on adaptive immunity, further increasing the risk of RRTI. Alongside functional immune immaturity, the anatomical immaturity of the pulmonary system increases the risk of RRTI, which makes antigen-rich environments such as day care nurseries a significant challenge.
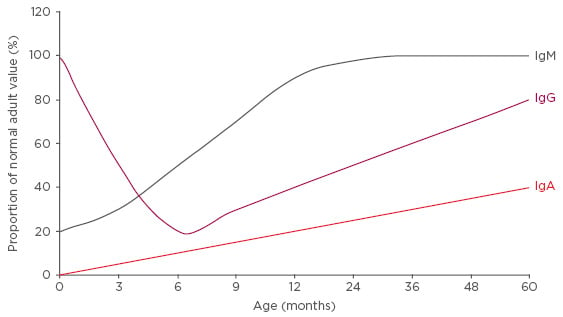
Figure 2: Immunoglobulin capacity as a proportion of adult values by age.
Ig: Immunoglobulin.
RRTI may occur in the upper airway (otitis media, mastoiditis, pharyngo-tonsillitis, adenoiditis, and rhinosinusitis) or the lower airway (bronchitis/bronchiolitis, tracheitis, and pneumonia). At times, infections may involve the entire airway, such as in the infectious processes of rhinovirus and influenza virus. URTI represent the majority (80−90%) of RTI;21 RTI are often self-limiting and those that are not easily managed are usually lower respiratory tract infections (LRTI), which are more severe and difficult to treat.
A universally agreed definition of RRTI is currently lacking. Six or more URTI, or at least one infection per month during autumn and winter, in children >3 years of age, or eight or more episodes per year in children <3 years old, have been proposed as suitable definitions. For LRTI, the lower rate of ≥3 episodes per year has been proposed. Both the aforementioned proposals relate to patients without immunological deficiency or functional/anatomical alterations. A more generalised concept of RRTI being present is when a child displays a higher frequency of infections compared to peers from the same age cohort and environment.
Twenty-five percent of the morbidity experienced by children due to RTI occurs during the first year of life, concurrent with the immunological nadir that accompanies gut closure to IgG. Another 18% of morbidity occurs in children aged 1–2 years, during which time the spike of antigen exposure due to attendance of nurseries generally occurs. Furthermore, 19% of all child deaths from RTI occur within the first 5 years of life.22,23
Aetiology
The vast majority (90%) of RRTI are caused by viruses. Common causal agents include respiratory syncytial virus (RSV), rhinovirus, and viral influenza. The remaining 10% of infections involve bacteria but generally also have a viral element, manifesting as joint viral/bacterial superinfections. Bacterial causal agents include Streptococcus pneumoniae, Haemophilus influenza, Staphylococcus aureus, and Klebsiella pneumoniae, amongst others.
Previously, RSV was considered the most prevalent RTI pathogen during childhood. However, data from studies using molecular techniques such as the polymerase chain reaction indicate that rhinovirus is in fact the predominant pathogen.24 Rhinovirus finds a permissive environment for replication in the adenoids following entry through the upper airway, and from this site can initiate both URTI and LRTI.
The functional immune immaturity of infants combines with multiple risk factors to precipitate respiratory infections, including overexposure to antigens in nurseries, allergies, siblings, seasonality, environment, and lack of breast feeding. A study in the Netherlands directly demonstrated the effect of targeting risk factors rather than the disease itself. Breast feeding for ≥6 months was associated with a reduced risk of LRTI in infants ≤4 years of age, compared with children who were never breastfed (adjusted odds ratio: 0.71; 95% confidence interval [CI]: 0.51–0.98).25
The burden of respiratory disease is borne both by the child and their family, leading to an overall economic burden, school and work absenteeism, misuse of antibiotics and associated resistance, decline in pulmonary function, and impact on QoL.26 In a Chinese study, children aged 2−7 years affected by RRTI had significant deficits in physical, emotional, social, and school functioning (p<0.05).27 LRTI are more serious and are associated with an increased risk of wheezing or asthma, hospitalisations, and death.26 Hospitalisation with pneumonia is a serious condition in children and adolescents, with 21% of patients requiring intensive care. Approximately 50% of hospitalisations in this age group are caused by a single virus, with an additional 15% being caused by viral–viral coinfection. Similar patterns are seen in the <2 years age group (approximately 80% single and co-viral infections) and the 2−4 years age group (approximately 80% single and co-viral infections). More than half of children aged <2 years hospitalised due to pneumonia tested positive for RSV or rhinovirus, while just under half of those aged 2−4 years tested positive for RSV and/or rhinovirus.27
The predominance of viral rather than bacterial aetiology in RTI is most marked in preschool children, with 95% of infections being viral in this age group. In school age children, the proportion drops to approximately 85%, and in adults it is 80%. The high rate in preschool children is likely to be at least partially mediated by a Type 2 T helper (Th2) cell predominance in the functionally immature immune system. Th2 cells are involved in defence against parasites but have more limited activity against viruses. In addition, Th2 cells have a role in allergy. The combined effect of Th2 dominance and viral RRTI may lead to wheezing, which increases the risk of developing asthma. Furthermore, in a vicious cycle of sorts, in those patients who do develop asthma, RTI are a major trigger for exacerbations.28-30 The extremely high predominance of viral infections in preschool children further underlines the need to avoid antibiotic use in this population unless absolutely necessary.
Recent data have shown that the nasopharyngeal microbiome, which is colonised during the first year, affects both the severity of LRTI and asthma.31 Early colonisation of the nasopharynx was by Staphylococcus or Corynebacterium, followed by Alloiococcus; transient appearance of Streptococcus, Moraxella, or Haemophilus was associated with increased viral infections. The presence of these virus-associated bacteria was linked to inflammatory processes, an increased chance of LRTI, and an increased risk of wheezing or asthma. Antibiotic use was also associated with these pathogenic species, providing a possible mechanism behind the reported association between early antibiotic use and asthma.
CAN THE MANAGEMENT OF RECURRENT RESPIRATORY TRACT INFECTION AND CHRONIC RESPIRATORY DISEASES BE IMPROVED?
Knowledge and detection of risk factors is the first step in the prevention of RRTI. Education of parents, reduced exposure to environmental pollutants, active immunisation, and the use of immunomodulators may all improve RRTI prevention.32,33 Cochrane systematic reviews represent the gold standard for evidence-based medicine.34 In 2012, an update on the first 2006 Cochrane review on immunostimulants for the prevention of RTI was published.35,36 The goal of the analysis was to assess the safety and efficacy of immunostimulants administered to children as RTI prophylaxis therapy. Data from randomised trials on the use of immunostimulants and immunomodulators for the control of RTI in children ≤18 years of age were included. The primary outcome of interest was the number of acute respiratory infections during the period of analysis. Children with allergies, asthma, anatomical malformations, Down’s syndrome, or other forms of immunodeficiency were excluded.36
Of the 764 studies identified, 61 studies were included, comprising 4,000 patients. Of the included studies, 45 lasted <6 months, 35 lasted 6 months, and 2 lasted >6 months. The included studies were evaluated for quality (Level A, B, or C) before data extraction. The extracted data revealed significant heterogeneity and variability in the trials. Immunostimulators were shown to reduce RTI (-1.24; 95% CI: -1.54–[-0.94]), with a difference in rates of -39% (95% CI: -46.37–[-31.31]). The overall quality of evidence for any immunostimulant versus placebo was moderate and depended on the number of URTI in the control group.36
Data from studies on bacteria-derived immunomodulators had the least heterogeneity and variability, particularly those studies investigating the effects of the bacterial lysate OM-85. Of the 12 OM-85 studies assessed, all were of ≥6 months’ duration. Four OM-85 trials were the only A-rated studies amongst bacterial immunomodulators, with the remaining 8 OM-85 studies rated quality-level B. The reduction in the total number of acute respiratory tract infections as compared to placebo was 35.90% (95% CI: -49.46–[-22.35]).
OM-85 Prophylaxis in High-Risk Children
OM-85 prophylaxis was assessed in a 6-month study of children aged 3−5 years with a history of RRTI and subnormal IgG subclasses (N=54).37 The frequency of subnormal IgG subclasses was high in this population (78%) and OM-85 significantly reduced the incidence of acute RTI by approximately 40%. A separate randomised double-blind trial investigated the use of OM-85 in the city of Chihuahua, Mexico, in children aged 1−12 years (N=54) with a history of RRTI. The study ran for 12 months and encompassed two 3-month dosing schedules of OM-85 at 3.5 mg/day for 10 days/month (standard dosing) or placebo. OM-85 prophylaxis resulted in a lower use of antibiotics from Month 2 onwards, including during the winter months when extreme weather was prevalent, which was mirrored by a reduction in the number of RTI.38
Overexposure to respiratory pathogens is common in orphanages. From the researcher’s perspective, orphanages also have the advantage of providing a homogenous environment for study participants. A randomised double-blind placebo-controlled trial investigated the use of OM-85 in girls aged 6−13 years (N=200) living in an orphanage. Participants received either OM-85 prophylaxis at the aforementioned standard dose or placebo. During the 6-month follow-up, which encompassed the winter season, participants who received OM-85 had a 50% reduction in the incidence of RTI. All untreated children required antibiotic therapy, while approximately 50% of patients who received OM-85 prophylaxis also received antibiotics. The relative risk reduction increased along with increased frequency of RTI, with a risk reduction for ≥3 RTI of 0.2 versus placebo, indicating an 80% reduction in risk. As well as representing a reduction in the burden of illness for individuals, including reduced absences from school, these changes likely resulted in a significant cost saving for the institution.39 Similarly, in an open-label multicentre study carried out in an orphanage, OM-85 decreased infections, the use of antibiotics, and school absenteeism when given in the acute phase of infection. In addition, OM-85 had an increased protective effect in those patients who had a history of more frequent infections.40
As with all medical interventions, safety of immunomodulators is a key consideration. Data from the 2012 Cochrane review show that OM-85 has a risk difference of close to zero compared with placebo (0.01; 95% CI: 0.01–0.03).36 The documented adverse events were mild in nature and consisted of gastrointestinal events, including diarrhoea, or skin reactions.
Efficacy in Acute Respiratory Tract Infections
The aforementioned data, as well as similar studies such as by Chen et al.,41 in children in remission from CRS, show that OM-85 has a prophylactic effect in RRTI. Though its primary role is in prophylaxis, open-label and controlled studies have shown that OM-85 accelerates the recovery of patients when used during the acute phase of the infectious process. In a randomised double-blind trial in children aged from 18 months to 9 years (N=56) with subacute rhinosinusitis, therapy with OM-85 reduced convalescence (15.38±8.91 days versus 20.28±7.17 days) and promoted faster recovery (5.56±4.98 days versus 10±8.49 days) compared with placebo with these children.42 Similar results showing both curative and prophylactic effects of OM-85 were found in another study on acute episodes of CRS in children.43
Immunogenicity
Vaccination is the primary preventative measure against viral infections. Safety, efficacy, and immunogenicity were investigated in a 6-month prospective, randomised, single-blind study in children (N=68) who received OM-85 in combination with inactivated influenza vaccine. Incidence, mean number, and prevalence of RTI were all significantly lower in children who received OM-85 prophylaxis compared with vaccine prophylaxis alone. Missed school days and the number of antibiotic courses were also significantly lower in the combined prophylaxis group. Furthermore, there was no difference in anti-inactivated influenza vaccine IgG and IgM, indicating that OM-85 did not interfere with the vaccineimmune response.44
IMMUNOMODULATORS IN RHINOSINUSITIS GUIDELINES
In the current European position paper on adults with rhinosinusitis, OM-85 prophylaxis in CRS without nasal polyps has an evidence level of 1B and recommendation grade A, the second highest evidence level, below only nasal steroids.20 Pan-American guidelines state the beneficial effect of preparations of bacterial lysates in adults to reduce the proportion of recurrence in viral and bacterial rhinosinusitis, and that the benefits of bacterial lysates outweigh the risks. These guidelines also recommend bacterial lysate therapy for CRS without polyps, though sufficient evidence for CRS with polyps is lacking.45 There are currently no guideline recommendations for the use of bacterial lysates in children. However, as detailed previously, data from diverse geographical regions and paediatric populations suggest a benefit, and Brazilian guidelines mention the benefit of bacterial lysate therapy in children with RRTI that may lead to CRS.46
CONCLUSION
Children and fragile adults are particularly vulnerable to the development of RRTI, as well as chronic conditions such as CRS. As the definition of recurrence varies, it can be a challenge for physicians to interpret research and choose the optimal therapeutic option for their patients. Treating each case as an individual, both from a pathological and patient-focussed standpoint, can help guide treatment. When assessing RRTI, risk factors should be taken into account, the importance of the URT microbiome should be considered, and rational and minimal use of antibiotic should be a key consideration. Given the downstream consequences of RRTI in terms of increased asthma risk, hospitalisation, morbidity, and mortality, appropriate prophylaxis remains a major unmet need. Behavioural interventions, targeted medical and surgical interventions, and non-specific immunostimulation or immunomodulation should all be considered in the context of individual patient circumstances. Immunomodulators should be a significant part of a physician’s preventative strategy, alongside a proper medical diagnosis, environmental control, effective nutrition, and appropriate use of other medications. The bacterial lysate immunomodulator OM-85 has the most robust and consistent evidence base. In RRTI, OM-85 has been shown to reduce incidence, convalescence, and the use of antibiotics, as well as being more effective in children with risk factors, such as those who attend nurseries or live in orphanages. It has also proved to be tolerated and effective in combination with the influenza vaccine and to be able to speed recovery and decrease convalescence during the active management of rhinosinusitis.








