Meeting Summary
The objective of this symposium was to provide an overview of Type 2 inflammation in asthma. The speakers covered the pathophysiology of Type 2 asthma, its heterogeneity, the associated economic burden, and methods for recognising Type 2 inflammation in severe asthma patients in clinical practice.
Asthma is a heterogenous disease and multiple phenotypes are common among patients. Type 2 asthma is so named because it is associated with Type 2 inflammation and typically includes allergic asthma and moderate-to-severe eosinophilic asthma, Prof Canonica explained. By contrast, non-Type 2 asthma commonly has an older age of onset and is often associated with obesity and neutrophilic inflammation.
Prof Diamant highlighted the scale and severity of uncontrolled persistent asthma. Globally, an estimated 420,000 people die of asthma every year, and many more have uncontrolled disease, putting them at risk of persistent airway inflammation and eventual lung decline. Patients may not recognise that their disease is uncontrolled, despite exacerbations and the impact of their asthma on daily activities. Prof Diamant described the impairments to health-related quality of life and the associated costs of uncontrolled asthma.
Prof Dahlén outlined how new predictive biomarkers will be needed to identify the type of asthma an individual patient has. No single biomarker will provide sufficient information, and as such, in the future, profiles of many markers will need to be integrated to produce subgroup-specific profiles for use in personalised medicine. He described ongoing research into protein arrays and lipid mediators in urine, and how cluster analysis and pattern recognition, with the aid of artificial intelligence, will form the basis of future diagnostic tools. Prof Canonica explained that an understanding of the mechanisms of asthma is important in achieving better symptom control. IL-4 and IL-13 are key players in the pathobiology of uncontrolled persistent asthma (IL-4 in inflammation and IL-13 in airway remodelling), but their roles overlap. The heterogenous nature of Type 2 asthma can make it difficult to diagnose; therefore, focussing on a single biomarker is likely to leave some patients sub-optimally controlled.
Introduction
This symposium aimed to establish the need for a greater understanding of the mechanisms of disease driving uncontrolled persistent asthma. The disease is heterogenous in nature and multiple phenotypes of Type 2 asthma are common among patients. The speakers reviewed scientific evidence supporting the role of key mediators of Type 2 inflammation in uncontrolled persistent asthma and discussed new and emerging methods for identifying patients with Type 2 asthma in clinical practice. The burden of uncontrolled persistent asthma is substantial for patients and society. An audience poll found that most participants thought that 45% of European asthma patients have uncontrolled persistent asthma, which Prof Canonica said was a reasonable estimate.
Defining Severe Asthma: Understanding the Evolving Profile of Uncontrolled Persistent Asthma
Professor Zuzana Diamant
Asthma is a prevalent disease that is not fully manageable. In 2016, the Global Burden of Disease study estimated that 300 million individuals worldwide have asthma,1 which causes 420,000 deaths per year (Figure 1).2 However, Prof Diamant stated that this number of fatalities may be an underestimate.
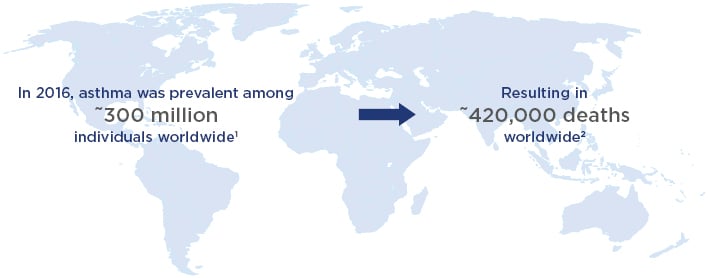
Figure 1: Asthma: A global public health concern.1,2
An understanding of the pathophysiology of asthma is essential for disease management.Airway inflammation is a key feature of uncontrolled asthma3,4 and is associated with airway hyper-responsiveness and remodelling,5 in which the lumen becomes narrowed and the airway wall thickened compared with controlled asthma.3 These features (inflammation, fixed obstructive airway through hyper-responsiveness, and airway narrowing) interrelate and have been repeatedly shown to be more prominent in uncontrolled compared with controlled asthma.6,7 Airway narrowing is mostly reversible, Prof Diamant said, but persistent airway inflammation is associated with eventual lung function decline.6,7
Over the past 20 years, attempts have been made to define severe asthma.8 The latest recommendations on the identification, evaluation, and treatment of patients with severe refractory asthma were published in a 2014 joint consensus from the American Thoracic Society (ATS) and the European Thoracic Society (ETS).9 The publication distinguishes between severe asthma, which requires treatment with high-dose inhaled corticosteroids (ICS), and asthma that is uncontrolled despite this therapy. Prof Diamant said it is important to identify and address factors such as adherence to therapy, comorbidities, and chronic environmental exposures before making the diagnosis of uncontrolled asthma. This type of asthma remains uncontrolled despite intensive pharmacotherapy but includes asthma that is controlled for at least 6 months with high-dose oral corticosteroids (OCS).
According to the 2018 guidelines from the Global Initiative for Asthma (GINA),10 asthma control should be assessed in terms of symptom control and future risk. Symptom control includes daytime symptoms, night-time awakenings due to asthma, the need for relief medications more than twice a week, and limitations on activity because of asthma. Future risks include the risk of exacerbations, medication side effects, and airway obstruction or lung function decline. Prof Diamant stressed that too many people (40–70%)11 experience uncontrolled asthma and are exposed to these risks.
The GINA guidelines10 are based on research that emphasises the importance of asthma control. One study found that poor asthma control almost doubles the risk of hospitalisation for asthma exacerbation;12 another study found that exacerbations lead to increased symptoms and decreased lung function compared with controlled asthma.7 A recent asthma exacerbation increased the risk of future exacerbations 6-fold compared with patients without a recent severe exacerbation (odds ratio [OR]: 6.33; 95% confidence interval: 4.57–8.76) in a 2007 study;13 the OR was only slightly lower when corrected for physician-adjusted or GINA-adjusted disease severity.
OCS are associated with significant health problems. Patients who have received ≥4 OCS prescriptions are significantly more likely to experience adverse effects (AE), such as osteoporosis, hypertension, Type 2 diabetes mellitus, and gastrointestinal ulcers and bleeds (OR: 1.21–1.44, depending on the AE), compared with patients who have not taken OCS.14 Therefore, the GINA stepped approach to treatment10 reserves OCS for the last step of treatment, after the use of drugs such as ICS, beta-2 agonists, and theophylline.
The personalised approach to asthma control, recommended by GINA,10 requires physicians to assess and diagnose the disease, adjust treatment, and review the patient’s response. This includes addressing comorbidities and modifiable lifestyle factors and taking patient preferences into account. Treatment response should be reviewed on a regular basis.
Despite such guidelines for physicians, a substantial number of patients continue to live with uncontrolled disease and many do not realise that their asthma could be improved. The online survey REALISE (REcognise Asthma and Link to Symptoms and Experience)15 included 8,000 patients from 11 European countries. Overall, only 20% of respondents had fully controlled disease, but 80% of patients who had experienced acute exacerbations in the past 12 months considered their asthma to be controlled. Similarly, in the Europe and Canada (EUCAN) Asthma Insight and Management (AIM) survey,16 which included telephone interviews with 2,003 patients, over half of the group (51%) reported acute treatment of asthma in the past year, such as hospitalisation or unscheduled emergency visits. On the other hand, 77% of the group perceived their asthma to be well or completely controlled.
The LIAISON study17 included 8,111 patients from 12 European countries. The study used the Asthma Control Questionnaire (ACQ) and the MiniAsthma Quality of Life Questionnaire (MiniAQLQ) and found that asthma control was suboptimal in 56.5% patients. Quality of life was related to asthma status and deteriorated as control of the disease lessened, Prof Diamant said.
Uncontrolled asthma impairs health-related quality of life. For example, sleep quality and quantity are decreased and sleep disturbances are more frequent and severe.18 Many patients also experience impairment of outdoor and other daily activities,19,20 and have increased symptoms of anxiety and depression that can complicate disease management.21 Poor asthma control has a negative impact on an individual’s wellbeing.
On a major scale, Prof Diamant said the health economic consequences of uncontrolled asthma are shocking. One report estimated that persistent asthma represented a €19.3 billion economic burden in Europe in 2010.22 Most of these costs were due to uncontrolled asthma, with per-patient costs four-times higher than costs associated with controlled asthma. Similarly, Demoly et al.20 found poorly controlled asthma was associated with a substantial use of healthcare resources and a negative overall impact on work productivity and daily life.
Prof Diamant concluded by stating that a minority (5–10%) of patients with severe asthma account for around half of the total healthcare costs for asthma. She said a greater understanding of uncontrolled persistent asthma and a more personalised approach to asthma management are needed to achieve optimal disease control.
Type 2 Asthma: What is it? What Mediators Drive it?
Professor Sven-Erik Dahlén
An understanding of Type 2 asthma will underpin future developments in precision medicine, Prof Dahlén said. Asthma differs from other inflammatory diseases in that it involves not only chronic inflammation driven by immunological mechanisms but also the mechanisms leading to smooth muscle constriction and hyper-reactivity of the airways. Today, and in the future, combination therapy that addresses both components of the pathophysiology is necessary to treat both aspects of the disease.
There are four components of asthma: bronchoconstriction, airway inflammation, airway hyper-responsiveness, and chronic remodelling of airways. Inflammatory mechanisms act on all components with an interplay between several different cells and mediators, Prof Dahlén said.
Today’s concept of Type 2 inflammation builds on immunological research from the 1980s into control mechanisms in the immune system. It was known that antigens interact with dendritic cells and, as a result, T cells are activated, causing them to differentiate and stimulate different immune pathways. Th2 cells were found to be prominently activated by allergic reactions, leading to allergy, eosinophilia, and the release of IgE and IgG1. Th1 cells, which release IFN-γ and TNF-α, were found to be more involved in cell-mediated immunity. Later, the autoimmunity pathway driven by IL-17 and IL-22 was recognised, along with the anti-inflammatory pathway driven by IL-10 and TGF-β.23
Most of this work was carried out in mice and 10–15 years ago its relevance in humans was doubted, Prof Dahlén explained. The breakthrough came 10 years ago when several groups started researching the mechanisms of human asthma. A seminal study carried out in San Francisco, California, USA, took brushings of airway epithelium in asthma patients and healthy controls and found that a Th2 profile similar to that previously observed in mice was prevalent in asthma patients.24 The Th2 profile was associated with expression of IL-5 and IL-13 and subsequent work showed that this profile was also associated with a good therapeutic response to inhaled steroids, which are still the mainstay of asthma treatment.
Allergic asthma is characterised by a Th2-dominated immune response. Inhaled allergens lead to increased serum IgE levels; IgE binds to mast cells and basophils, which secrete histamine and lipid mediators that cause bronchoconstriction and activate other inflammatory cells. Mast cells and basophils also secrete IL-4 and IL-13, causing plasma and cell extravasation and recruitment of eosinophils, the key cells involved in the inflammation.25 An unmet need still present in asthma treatment is the need to limit mucus secretion driven by IL-13, Prof Dahlén said.
Eosinophils have a key role in Type 2 inflammation in asthma. IL-5 promotes eosinophil differentiation in the bone marrow; mature eosinophils migrate to the circulation; and Type 2 cytokines, including IL-4 and Il-13, attract the eosinophils to the airway. The inflammatory response is amplified by the recruited eosinophils via a positive feedback loop, driven by IL-4. The inflammatory mediators secreted by eosinophils and other inflammatory cells lead to tissue damage, creating a cycle of chronic inflammation (Figure 2).26-28 Prof Dahlén said there is a suggestion that IL-4 and IL-13 may also drive airway hyper-responsiveness.
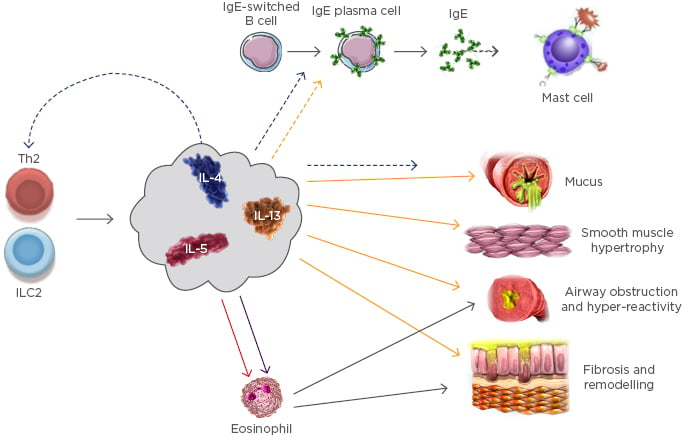
Figure 2: Key cells and mediators in Type 2 asthma.26
ILC2: Type 2 innate lymphoid cells .
Prof Dahlén’s group is currently studying samples of human lung tissue. When exposed to IL-13 in vitro, lung tissue has an increased contractor response to histamine and leukotrienes; IL-4 gives a similar response, suggesting that IL-4 and IL-13 upregulate airway sensitivity. Prof Dahlén noted that both IL-5 and IL-17 have been thought to impact airway responsiveness, but they were not shown to have this effect in his own research.
Asthma is now established as a heterogenous disease composed of many phenotypes (Figure 3).29,30 It is classified as either Type 2 asthma, which includes allergic and eosinophilic asthma, or non-Type 2 asthma, which is associated with obesity and smoking and driven by neutrophils. However, there is overlap in the mechanisms driving these two types of asthma;31 for example, IL-33 and IL-25, released in non-Type 2 inflammation, stimulate Type 2 innate lymphoid cells (ILC2) to release IL-13 and IL-4 and activate Type 2 inflammation.3
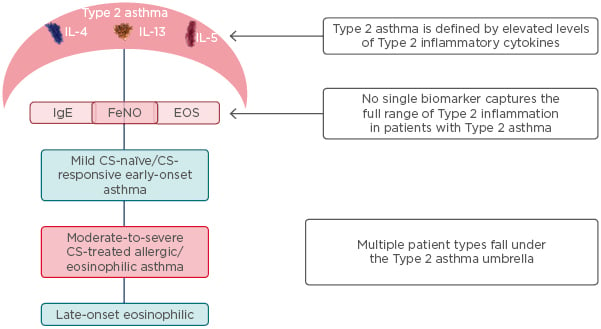
Figure 3: Type 2 asthma includes a wide range of patients with different phenotypes.29,30
CS: corticosteroid; EOS: eosinophil; FeNO: exhaled nitric oxide fraction.
For this understanding to translate into precision medicine in the clinic, Prof Dahlén said effective predictive biomarkers will be needed to identify which type of asthma the patient has and what treatment is appropriate. To date, the key established biomarker of Type 2 inflammation is the number of eosinophils in blood and sputum samples. IgE levels may be a marker for allergic asthma. In experimental work, periostin appears to be a promising biomarker, but clinical measurements using current assays have so far been less convincing. Exhaled nitric oxide fraction (FeNO) is emerging as an alternative biomarker for Type 2 inflammation, he said.
In a 2009 study, patients with allergic asthma inhaled low doses of allergen on seven consecutive weekdays.32 This allergen exposure caused significant increases in airway hyper-responsiveness, which were detected by measuring FeNO (mean±standard deviation: 46±31 ppb before versus 73±46 ppb after). This inflammation was blocked by ICS but not affected by beta-antagonists.32 Other studies have also shown a relationship between increased FeNO and increased asthma morbidity,33 and Prof Dahlén suggested that combining monitoring FeNO levels with eosinophil measurements in blood may be a better biomarker than eosinophil count alone.
However, more precise biomarkers are needed. Prof Dahlén suggested that no single biomarker will ever be sufficient and that, in the future, profiles of many markers will be integrated with artificial intelligence. The Human Protein Atlas34 includes >25,000 antibodies targeting 17,000 proteins. Prof Dahlén and colleagues constructed an array of 177 proteins implicated in inflammation and asthma. They applied the array to plasma samples taken from 434 patients as part of the pan European U-BIOPRED study,35 for which extensive clinical and physiological data were available. Measuring the expression of the array proteins in patients’ blood samples identified six distinct patient clusters; the largest was a typical Type 2 inflammation cluster. Prof Dahlén said the work was at an early stage, but he predicted that this approach, looking for the protein ‘fingerprints’ in blood samples, will be used in the clinic in the future.
Another avenue of research is the measurement of lipid mediators in urine. Lipid mediators, such as prostaglandins and leukotrienes, are part of the inflammation response and Prof Dahlén’s group has developed a method for quantifying 26 lipid mediators and their metabolites.36 They have found that urinary leukotriene E₄ (LTE₄) levels are higher in severe compared with mild asthma, and higher in asthma patients than in healthy controls. There is an overlap in LTE₄ levels between groups, but extreme value analysis found that individuals with the highest levels of LTE₄ also had high eosinophil counts in the blood and sputum, suggesting that LTE₄ is a marker for Type 2 inflammation.36
Clustering analysis of the profiles of the main lipid mediator metabolites in urine found five subphenotypes, the biggest of which was Type 2-driven. Prof Dahlén said that, since this subgroup was identified solely on the basis of 11 metabolites in urine, it may be possible in the future to create a dipstick test for this subtype of asthma.
In summary, Prof Dahlén said that more needs to be discovered about Type 2 asthma, which is an umbrella term for asthma with inflammation driven by Type 2 cells and mediators; the list of involved mechanisms is increasing as research progresses. Type 2 asthma includes several subphenotypes with different clinical characteristics, which are likely to respond to different future treatments. Current biomarkers (blood and sputum eosinophil number, FeNO, and total IgE) are used to indicate Type 2 inflammation in asthma, but research is ongoing to find improved biomarkers for subphenotypes. Type 2 asthma classically responds to ICS, but this therapy is insufficient to provide optimal control in severe cases and OCS have significant side effects. Future goals include improved diagnosis of the subphenotypes of Type 2 asthma along with new precise treatments with a lower side effect profile than at present.
Identifying Type 2 Asthma in Clinical Practice
Professor G. Walter Canonica
Approximately 50–70% of asthma patients have Type 2 asthma,37,38 which is characterised by Type 2 inflammation. This group typically includes allergic asthma, exercise-induced asthma, and late-onset eosinophilic asthma. By contrast, non-Type 2 asthma commonly has an older age of onset, is associated with obesity and smoking, and is neutrophilic, smooth muscle-mediated, and paucigranulocytic.31
An understanding of the mechanisms of asthma is important to achieve better disease control. In the clinic, patients are evaluated according to measures of control (exacerbations, forced expiratory volume in 1 second [FEV₁], and use of ICS/OCS), quantifiable biomarkers (eosinophils, FeNO, and IgE), and patient characteristics (allergies and comorbidities). Recently, the need to check for nasal polyps and allergic rhinitis was recognised; an Italian registry39 found that 60% of severe asthma patients had a diagnosis of allergic rhinitis. The registry also found that 62% of patients have taken OCS, and that eosinophil counts were higher than desirable, Prof Canonica said.
The audience was asked whether they routinely use and rely on spirometry (FEV₁ and FEV₁/forced vital capacity ratio) in the clinical management of patients with uncontrolled persistent asthma. The poll found that 9% of the audience members use spirometry 25% of the time, and a further 9% use it 50% of the time. This is broadly in line with the results of an Italian study that found that only 69.5% of patients with doctor-diagnosed asthma had received spirometry testing as part of their assessment.40 Prof Canonica said he routinely uses spirometry for patients with uncontrolled persistent asthma and he urged the audience to use this ‘easy parameter’ more frequently, especially considering that there is a greater chance of misdiagnosis when spirometry is not used to measure lung function.
When asked whether they use IgE measurements to manage this patient group, Prof Canonica was encouraged that >40% of audience members always relied on IgE measurements; however, 17% said they used it only 25% of the time. On the routine use of eosinophil measurements, around one-third (34%) of the audience said they used it either 25% or 50% of the time in this patient group.
Asthma was previously defined as being Th2 or non-Th2. The current distinction between Type 2 and non-Type 2 asthma acknowledges that cytokines associated with Th2 responses are broadly secreted by numerous cell types beyond the originally-described adaptive Th2 immune response.26 These include ILC2, which are part of the innate immune system and do not require antigen interaction to secrete Type 2 cytokines. Adaptive and innate immune systems work independently, Prof Canonica said, but both produce Type 2 cytokines IL-4, IL-13, and IL-5.
IL-4 plays a central role in inflammatory cell activation (Th2 and ILC2), mast cell recruitment, and initiation of the signalling that drives asthma pathophysiology. IL-4 secretion mediates Th2 cell differentiation from naïve Th cells, can induce ILC2 proliferation, and is essential for the maintenance of the ILC2 phenotype. IL-4 promotes the downstream secretion of Type 2 cytokines via Th2 and ILC2; it also recruits inflammatory cells and induces IgE production.26,41,42
IL-13 is a key mediator of airway remodelling. It causes goblet cell hyperplasia and mucus overproduction, as well as an increase in subepithelial collagen deposition through the transformation of bronchial fibroblasts to myofibroblasts.43 IL-13 promotes airway epithelial cell expression of inducible nitric oxide synthase, which increases the levels of FeNO.43,44 The effects caused by IL-13 contribute to a notable increase in bronchial hyper-responsiveness;43 IL-13 also contributes to asthma pathobiology through effects on the smooth muscle.44-46 In vitro experiments suggest that IL-13 can increase cell contractility to acetylcholine in smooth muscle and reduce beta-agonist-mediated relaxation.45
The Type 2 cytokines IL-4, IL-13, and IL-5 are secreted by adaptive Th2 helper cells, innate ILC2 cells, mast cells, and eosinophils.26 IL-5 promotes eosinophil differentiation and maturation,26,27 while IL-4 and IL-13 promote trafficking of eosinophils to the airway.26-28 Recruited eosinophils further amplify the Type 2 inflammatory response via a positive feedback loop by secreting more IL-4, and the inflammatory mediators secreted by eosinophils and other inflammatory cells lead to tissue damage, creating a cycle of chronic inflammation.26
The distinct and overlapping roles of IL-4 and IL-13 make them key players in the pathobiology of uncontrolled persistent asthma, Prof Canonica said. They induce inflammatory and structural changes that are characteristic features of this heterogeneous disease. The heterogenous nature of Type 2 asthma can make it difficult to diagnose and fully control. Prof Canonica said that no single biomarker could capture the full range of Type 2 inflammation and that focussing on a single biomarker is likely to leave some patients suboptimally controlled.
Prof Canonica concluded that achieving optimal asthma control in patients with uncontrolled persistent asthma can be challenging. The full spectrum of Type 2 asthma patients can only be captured using multiple biomarkers of airway inflammation. The key to achieving optimal control in patients with Type 2 asthma is understanding the underlying mechanisms driving this heterogeneity. The future will be more complicated but will allow proper selection of patients for different treatments.
Conclusion
A personalised approach to the diagnosis and management of asthma is called for. Asthma is a heterogenous disease and multiple phenotypes are common among patients. Type 2 asthma is characterised by Type 2 inflammation and typically includes allergic asthma, exercise-induced asthma, and late-onset eosinophilic asthma. Understanding of allergic, eosinophilic, and mixed allergic/eosinophilic phenotypes has greatly advanced and may underpin new approaches to improve asthma control.
Novel biomarkers may further improve personalisation. Biomarkers based on pattern recognition of metabolites in urine samples, for example, will be applicable at the point of care and will help in patient selection for new biologic agents, especially when patients have atypical features of Type 2 asthma. New possibilities for patients will complicate the work of the physician. Prof Canonica concluded that professional education will be essential over the next few years if the potential of the new research is to be realised in the clinic.
SAGLB.AST.18.10.1370 November 2018








