Authors: David Price1, Arnaud Bourdin2
Affiliation: 1. Observational and Pragmatic Research Institute, Singapore
2. University of Montpellier, France
Disclosure: Price has advisory board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Viatris, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, and Thermofisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Viatris, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute PTE. Ltd.) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Viatris, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, and the National Health Service (NHS); payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Viatris, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, and Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Circassia, Mundipharma, Novartis, Teva Pharmaceuticals, and Thermofisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. Bourdin has received consultancy fees and speakers’ fees from AstraZeneca, Amgen, Boeringher Ingelheim, Novartis, GlaxoSmithKline, Sanofi Regeneron, and Chiesi; research grants from GlaxoSmithKline, Boeringher Ingelheim, and AstraZeneca; fees for participating on Data and Safety Monitoring Board from AB science; and is a member of the GINA scientific committee.
Acknowledgements: G. Walter Canonica, Professor of Respiratory Medicine at the Humanitas Clinical and Research Center and Humanitas University, Milan, Italy, and Liam Heaney, Clinical Professor at Queen’s University, Belfast, Northern Ireland, presented alongside Price at the AstraZeneca-sponsored symposium, ‘Proactive risk management: a novel approach to embedding OCS Stewardship into asthma care’, delivered on 5th September 2022 at the ERS International Congress 2022 in Barcelona, 4th–6th September 2022. Writing assistance for this article was provided by Rachel Danks, RSD Medical Communications Ltd, Gloucestershire, UK.
Disclaimer: The opinions expressed in this article belong solely to the named authors.
Support: This is an AstraZeneca-funded publication summarising an AstraZeneca-sponsored symposium and three posters selected by AstraZeneca. This article is intended for HCPs.
Citation: EMJ Respir. 2022;10[Suppl 2]:2-10. DOI/10.33590/emjrespir/10088956. https://doi.org/10.33590/emjrespir/10088956.
Meeting Summary
The continued inclusion of oral corticosteroids (OCS) in treatment guidelines, as well as the accessibility, familiarity, and relatively low cost of this therapeutic option compared with newer alternatives, has contributed to an ongoing overreliance on OCS treatments in severe asthma. This overuse continues despite accumulating evidence to demonstrate the detrimental long-term effects associated with even a short-term, low-dose course of OCS in this patient population.OCS Stewardship is a collaborative, systematic effort designed to protect patients with asthma from inappropriate OCS use through a series of patient- and physician-focused initiatives. Ultimately, OCS Stewardship aims to reduce OCS-related morbidity, lower the risk of OCS-related adverse events (AE), increase health-related quality of life, and reduce healthcare resource utilisation.
This article summarises data that were exhibited as part of the European Respiratory Society (ERS) International Congress held in Barcelona, Spain, describing a novel proactive risk-management approach to embedding OCS Stewardship into asthma care. The objectives of this meeting were to highlight the latest data demonstrating the need for OCS Stewardship in asthma; discuss approaches to assessing OCS exposure and OCS-related toxicities, and the rationale for systematic assessment of OCS toxicity in individual patients; and to consider practical tools to evaluate future risk of asthma exacerbations and OCS-related adverse effects. Also described in this article are three posters, which were presented during the same meeting, and provide further data to support OCS-sparing activities in severe asthma by AstraZeneca.
Introduction
Among the range of conditions for which systemic corticosteroids may be administered, respiratory diseases together account for approximately 70–80% of the total dose prescribed in the UK.1 Around the world, 20–60% of patients with severe or uncontrolled asthma receive long-term OCS therapy,2 and this treatment option continues to be included in national and international treatment guidelines.2 The reliance on OCS in the setting of severe or uncontrolled asthma persists despite the known long-term risks to patients.3,4 While the availability of new treatment options and guideline reform has brought about a dramatic reduction in the use of OCS for rheumatoid arthritis5 and Crohn’s disease,6 no similar transformation has yet relegated OCS to a treatment of last resort in asthma.
OCS Stewardship is a collaborative, systematic effort to protect patients with asthma from potential overexposure to OCS.3 Under the principles of OCS Stewardship, systemic corticosteroids should be used only when clinically appropriate at the lowest dose possible, with use and side effects monitored closely.7 In addition, both patients and physicians should be fully educated on the risks associated with OCS.3
In the AstraZeneca-sponsored symposium, ‘Proactive risk management: a novel approach to embedding OCS Stewardship into asthma care’, which was delivered on 5th September 2022 at the ERS International Congress 2022 in Barcelona, 4th–6th September 2022, the speakers evaluated the current state of OCS Stewardship, the impact of OCS use, and practical tools to enable risk prediction in asthma. Three posters exhibited at the same meeting also provided data to demonstrate the burden of OCS overexposure, as well as strategies to measure and mitigate the risks.
Oral Corticosteroid Stewardship: Where Are We Now?
The growing recognition of the risks of OCS in asthma is reflected in the Global Initiative for Asthma (GINA) guidelines, which have given decreasing prominence to OCS every year until 2022, when any mention of OCS in either the report or the treatment figures is explicitly qualified with the words “as [a] last resort.”8 Indeed, the 2022 guideline update states that OCS should only be considered after exclusion of other contributory factors and other add-on treatments, including biologics. Other national guidelines (including the Netherlands,9 Spain,10 Germany,11 France,12 Japan,13 and Italy14) also advise caution over the use of long-term OCS in patients with asthma. However, despite the warnings of recent guidelines, there remains a continued high dependency on OCS in this patient group. For example, data from the Italian Severe Asthma Network (SANI) show that over 64% of 437 patients with severe asthma in a real-world setting were taking long-term OCS at a mean dose (prednisone equivalent) of 10.7 mg/day,15 while a primary care database in the same country reveals that at least 76% of 284 patients received at least one OCS prescription during the first year following diagnosis of severe asthma.16 Similar data are reported in the UK, with the Severe Asthma Registry of 2,225 patients with severe asthma showing that 51.7% were receiving long-term OCS at a median dose of 10.0 mg/day.17
Further studies from the UK, USA, and Denmark report that only a small proportion of patients with severe asthma are referred to a specialist,18-20 despite such a referral resulting in a change of treatment in 55–68% of patients in one study.19 Referral rates are particularly low in the USA, where only 8% of patients with asthma managed in primary care were referred to a specialist within 24 months of treatment, despite 43% having uncontrolled asthma.19
Although the availability of targeted biologic therapies has reduced overall systemic corticosteroid use in rheumatoid arthritis and Crohn’s disease, total systemic corticosteroid use has increased over time in chronic obstructive pulmonary disease, where there is no biologic treatment available.21 In asthma, there has been a decrease in patients taking high-dose OCS in recent years following the introduction of biologics, but an increase in patients taking a relatively low OCS dose of less than 5 mg/day.21
The continued dependence on OCS, despite the availability of newer alternatives, including biologics approved for the treatment of severe asthma, is striking given the accumulated evidence demonstrating the risks of OCS among patients.3,22,23 Even intermittent or one-off use of OCS in asthma has been associated with OCS-related AEs, including depression/anxiety, Type 2 diabetes (T2D), pneumonia, and sleep apnoea (Figure 1).24 Several real-world studies have also shown that OCS use is associated with an increased risk of morbidity and mortality.25-27 In the UK, patients exposed to a cumulative dose of at least 10.0 g of systemic corticosteroids and followed for a median of 8.7 years were more than twice as likely to die as those exposed to less than 0.5 g.25 In South Korea, patients who received low-dose and high-dose corticosteroids had 1.84 (95% confidence interval [CI]: 1.69–2.00) and 2.56 (95% CI: 2.35–2.80) times higher mortality rates, respectively, than patients with corticosteroid-independent asthma.26 This study also showed a dose-dependent association with risk of mortality.26 Data from Sweden showed that regular OCS use was associated with an increased incidence of all-cause mortality compared with periodic and non-OCS use, with an adjusted hazard ratio of 1.34 (95% CI: 1.24–1.45).27
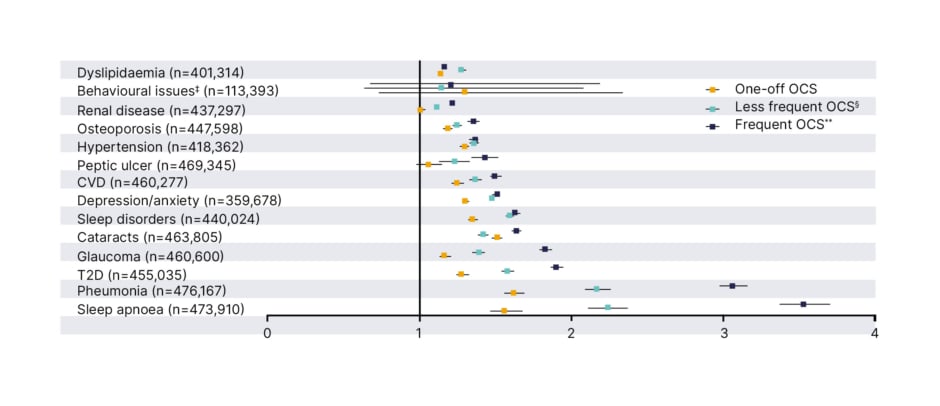
Figure 1: Hazard ratios* of oral corticosteroid-related adverse events for patients receiving intermittent oral corticosteroid versus patients who are oral corticosteroid naïve.†
*Hazard ratios were calculated using Cox regression analysis, adjusted for age, gender, BMI, smoking, and time-varying OCS prescriptions.
†OCS-naïve patients were matched with all patients receiving OCS prescriptions according to a 1:1 ratio.
‡Behavioural issues include diagnosis for distress, agitation, nervousness, emotional problems, irritable, and abnormal behaviour among patients aged <18 years.
§Patients with less frequent OCS use received all OCS prescriptions with a gap of ≥90 days.
**Patients with frequent OCS use received at least some OCS prescriptions, with a gap of <90 days, allowing for other prescription gaps to be ≥90 days.
Adapted from Heatley H et al.24
AE: adverse event; CVD: cardiovascular disease; OCS: oral corticosteroid; T2D: Type 2 diabetes.
Analysis of data from SANI showed that OCS use is more frequent in patients with asthma as well as other comorbidities.28,29 For example, patients with severe asthma and chronic rhinosinusitis with nasal polyps consumed OCS on twice as many days per year as patients without nasal polyps (161 days versus 79 days; p>0.02).28 Similarly, 69.4% of patients with severe asthma and bronchiectasis were taking long-term OCS compared with 42.5% of those patients without bronchiectasis.29 Furthermore, many of the comorbidities related to OCS, including anxiety/depression, osteoporosis, and T2D are associated with higher exacerbation rates.30
As well as the increased morbidity and mortality burden associated with OCS use in asthma,24-27 OCS-related AEs also have a substantial detrimental economic effect. In the SANI registry, the economic impact of the five most common OCS-related AEs (T2D, obesity, fractures, glaucoma, and chronic kidney disease) resulted in an additional expenditure of 41.5 million EUR annually in patients with severe asthma compared with patients without asthma, and 26.3 million EUR compared with patients with moderate asthma.31 The economic impact of OCS in severe asthma was also evaluated in a simulation study in six selected European countries, estimating the economic impact of comorbidities due to OCS use in naïve adult patients over a time horizon of 10 years.32 This analysis showed that the annual OCS-related comorbidities costs per prevalent severe asthmatic patient ranged from 1,712 EUR in Italy to 1,861 EUR in Poland, 2,158 EUR in Spain, 2,405 EUR in Slovenia, 3,907 EUR in Sweden, and 5,037 EUR in Belgium.32 Both analyses suggest that limiting OCS use in patients who are newly diagnosed severe asthmatic may be able to reduce the development of OCS-related comorbidities and associated costs.31,32
To address the issue of the overuse of OCS in asthma, activities to promote OCS Stewardship are gathering momentum around the word.4,33 In Italy, OCS Stewardship is being undertaken through medical education activities, including video lectures for the Patients’ Association, a media campaign undertaken in collaboration with SANI, and publication of a charter to improve patient care in severe asthma.34 Awareness campaigns have also been ongoing in Italy, including Asthma Zero Week (which campaigns for patients to access free consultancy in more than 40 asthma centres of excellence) and interviews on national television channels discussing OCS overuse in asthma. Finally, a consensus publication investigating OCS-sparing with biologics in severe asthma has been developed to encourage a change in approach from healthcare professionals.35
Assessing the Impact of Oral Corticosteroid Use: Moving Beyond Exposure
The use of systemic corticosteroids leads to multisystem toxicity in many patients.36 Although AEs associated with OCS use are traditionally considered to be dose-dependent or duration-dependent,36 the evidence increasingly suggests otherwise.37,38 There is, therefore, a need for a method of systematically measuring toxicity when patients are seen in routine care.
The Glucocorticoid Toxicity Index (GTI) was developed by 19 experts from 11 subspecialties as a tool to systematically capture and quantify glucocorticoid toxicity at the patient level.37,39 The GTI captures both cumulative worsening and aggregate change in toxicity over time. The cumulative worsening score (CWS) is an additive record of glucocorticoid-related toxicities experienced by a patient from baseline, with a higher CWS indicating a greater number of new toxicities encountered.38 The aggregate improvement score (AIS) is a measure of current toxicity, with a positive score indicating an increased total toxicity and a negative score reflecting reduced toxicity.38 The minimal clinically important difference for the AIS has been calculated to be 10.37 The GTI has been validated in both real-world experience and clinical trials across multiple indications including asthma, and provides a systematic way for glucocorticoid toxicity to be measured in routine clinical care.40
In a population of 101 biologic-naïve patients with severe asthma and significant OCS exposure (median cumulative prednisolone/prior year: 4,280 mg), the GTI score was shown to vary substantially among individual patients, indicating a wide distribution of toxicity.37 In addition, the GTI score correlated poorly with recent systemic steroid treatment, including maintenance prednisolone dose (r=0.26; p=0.01), cumulative exposure/prior year (r=0.38; p<0.001), and glucocorticoid boosts/prior year (r=0.25; p=0.01). On the other hand, GTI toxicity demonstrated stronger associations with patient-reported quality of life measures including the mini-Asthma Quality of Life Questionnaire (mini-AQLQ [r=−0.50; p<0.001]) and St. George’s Respiratory Questionnaire (SGRQ [r=0.42; p<0.001]).37
Following 12 months of anti-IL-5 treatment, daily prednisolone use among the same patients reduced from 11.7 mg to 6.7 mg (p<0.001) and the number of asthma exacerbations reduced from five to one over the period of treatment (p<0.001).38 However, AIS was found to vary widely around a mean of −35.7 (range: −165–+130), reflecting heterogeneous toxicity change at the individual patient level after treatment.38 Although the majority of patients had a significant reduction in toxicities, 30% experienced no change or a worsening in overall toxicity, while 39 of the 101 patients did not meet the minimal clinically important difference.38 Furthermore, toxicity change as measured by AIS showed no relationship with prednisolone reduction, baseline GTI toxicity, or change in quality-of-life measures.38 Thus, while biologic therapy resulted in substantial glucocorticoid reduction, this correlated poorly with short-term reduction in glucocorticoid toxicity, illustrating that established toxicity may not always improve with subsequent glucocorticoid reduction. This mandates the need for other approaches, including biologic therapies, to be initiated early before the manifestation of toxicity, and provides a rationale for including the reversal of systemic corticosteroid-induced toxicity as part of the concept of clinical remission in asthma.41
Practical Tools to Enable Risk Prediction in Asthma
Analyses using the GTI demonstrate that reducing or eliminating OCS even after relatively limited exposure does not necessarily reverse any long-term OCS-related harm once toxicity is embedded. On this basis, it is necessary to consider risk prediction and reduction in terms of future outcomes when initiating a given treatment. In particular, it is important to focus on quaternary prevention, which may be defined as the action taken to protect individuals from medical interventions that are likely to cause more harm than good.42
Modelling and measurement tools to predict risk have been successfully embedded in clinical medicine for a number of conditions associated with high morbidity and mortality, including cardiovascular disease (CVD),43,44 T2D,45 and osteoporosis.46 For example, the UK National Institute for Health and Care Excellence (NICE) recommends that a person’s 10-year risk of CVD should be assessed using the QRISK® (ClinRisk, Leeds, UK) assessment tool every 5 years (apart from people who already have CVD or are at high risk of developing it, or people aged 85 years or over).47 The QRISK model itself includes regular steroid treatment as a factor.43
Following the example of other therapy areas, where risk prediction models have proactively been built into clinical care, a similar model has been developed to evaluate the future adverse consequences of OCS in asthma, based on existing literature and databases, personalised risk factors, and hypothetical changes in future OCS use.48 This model also allows quantification of the effect of reducing OCS exposure on the future risk of common OCS-related comorbidities in asthma.
The model was based on data from 104,461 patients with asthma in the Optimum Patient Care Research Database (OPCRD), of whom 7,452 developed T2D.48 It allows the 20-year risk of T2D to be calculated based on known risk factors including age, sex, BMI, and smoking status, as well as future OCS use. As an example, a hypothetical White, 51-year-old female, who is an ex-smoker, with a BMI of 22.8 and anxiety/depression, but no hypertension or cerebro vascular disease/CVD, can be shown to have a 20-year risk of developing T2D of 8.86% using this model. However, if the same patient decreases their OCS use, this risk falls to 7.26%, while if they increase their OCS use, their risk rises to 13.41%.48 Thus, the relatively modest decrease in risk with a reduction in OCS use compared with the substantial increase in risk following an increase in OCS use provides a strong rationale to treat the patient early with OCS-sparing therapies, including biologics.
Real-word studies support the OCS-sparing properties of biologic treatments in asthma. For example, the GLITTER study of 1,992 patients with severe asthma and high OCS exposure across 19 countries from the International Severe Asthma Registry (ISAR) showed that initiation of biologic therapy led to a 69.2% reduction in exacerbations, a 57.3% reduction in asthma-related hospitalisations, and a trend towards a reduction in asthma-related emergency department visits (52.2%) compared with matched controls (matching was efficient by propensity scoring) who did not receive biologic treatment over 1 year of follow-up. Biologic initiators were 2.20-times more likely than non-initiators to have daily long-term OCS dose below 5 mg and more likely to achieve a high reduction (<75%) in total OCS dose (4.01-times).49
Analysis from the SANI registry similarly indicates that biologics reduce long-term OCS use.50 In a 3-year follow-up in a SANI single-centre study, 75% of 90 patients with severe eosinophilic asthma were found to respond to biologic therapy.50 Among these patients, the percentage on OCS dropped from 41% to 12% over the course of the study.50 In total, 43% of non-responders still needed OCS treatment despite shifting to another biologic after 12 months.50 However, switching treatment resulted in significant improvements in the incidence of AEs, as well as improvements in clinical measures of asthma (asthma control test [ACT] and forced expiratory volume after 1 second).50 This study suggests the benefit of switching treatment in case of a lack of response to a first biologic, although longitudinal real-world evidence of outcomes following biologic switching would be required to inform appropriate clinical decisions and ensure each patient receives optimal and timely treatment.51
The FIRE study was an observational, matched cohort study of patients with GINA Step 5 or uncontrolled GINA Step 4 asthma, who were eligible for both anti-IgE and anti-IL 5/IL-5 receptor therapy.8,52 In this study, both biologic treatments reduced the number of exacerbations.52 However, patients receiving anti-IL5/IL-5 receptors had a lower adjusted rate of post-therapy exacerbations (incident rate ratio: 0.76; 95% CI: 0.69–0.84) compared with those receiving anti-IgE.52 Thus, the ability to determine which patients are likely to benefit from different treatments will further the advancement towards personalised medicine in severe asthma. Furthermore, the timing of biologic treatment and a personalised approach to biologic selection may be critical to limiting OCS exposure and preventing toxicity.
Conclusion
In summary, long-term OCS use remains highly prevalent in patients with severe asthma,15-17 while relatively few patients with asthma are referred to a specialist.18-20 This is despite the evidence to show that referral for assessment by a specialist may actually reduce OCS use and improve outcomes.18-20
The continued dependence on OCS therapy in severe asthma persists despite clear evidence to show that even intermittent use of OCS is associated with AEs.24-27 Furthermore, comorbidities are common in severe asthma, and also contribute to higher OCS use and exacerbation rates, while the risk of comorbidities themselves also increases with frequency of OCS use.28,30 This morbidity burden leads to increased annual healthcare costs, placing a substantial financial responsibility on both patients and healthcare systems.31,32 OCS Stewardship describes a series of initiatives undertaken to protect patients with asthma from potential overexposure to OCS, and mitigate some of the effects described.3,4,33
Data from the GTI, developed to systematically measure glucocorticoid toxicity in routine clinical care, demonstrate that while biologic therapies result in substantial glucocorticoid reduction, this correlates poorly with short-term reduction in glucocorticoid toxicity.37-39 Furthermore, once toxicity has been established, it may not always improve with subsequent glucocorticoid reduction.38 This provides a strong rationale for initiating biologic therapies before toxicity appears.
New modelling tools in asthma demonstrate that reducing OCS exposure can lower the future risk of common OCS-related comorbidities,48 while real-word studies support the OCS-sparing properties of biologic treatments in asthma.49,50,52 However, the timing of biologic treatment and a personalised approach to biologic selection may be critical to limiting OCS exposure and preventing toxicity.
Veeva ID: Z4-50026; date of preparation: November 2022








