Meeting Summary
The global burden of respiratory diseases, particularly asthma and chronic obstructive pulmonary disease (COPD), continues unabated. Suboptimal management places a significant strain on both patients and urgent or emergency care services. With an ageing population in many countries, the demand for these services is set to increase further. At the same time, healthcare systems are striving to reduce their carbon footprint and achieve net zero emissions, as the healthcare sector is a significant contributor to carbon emissions worldwide. Although these two goals may appear contradictory, they need not be in conflict.
This article reviews an industry-sponsored symposium held at the European Respiratory Society (ERS) Congress 2024 in Vienna, Austria, in September 2024. The session addressed the urgent need to change the delivery model for respiratory healthcare in response to the increasing prevalence of respiratory diseases and the challenges posed by climate change.
Co-chair John Hurst, Professor of Respiratory Medicine at University College London (UCL), UK, underscored the importance of innovative solutions for managing respiratory diseases and highlighted the challenges faced by healthcare decision-makers. This was further elaborated on by Omar Usmani, Professor of Respiratory Medicine at Imperial College London, UK, who emphasised the importance of clinical choice. He stated that inhaled medicines, which form the cornerstone of treatment, should not be considered interchangeable. He also discussed ongoing efforts to maintain access to essential medicines by developing novel next-generation propellants (NGP) for pressurised metered-dose inhaler (pMDI) devices, which will reduce their carbon footprint to levels comparable with dry powder inhalers (DPI). Additionally, he described the European Chemicals Agency (ECHA) proposal to restrict a broad range of chemicals classed as per- and polyfluoroalkyl substances (PFAS). This precautionary measure would affect both current propellants in pMDIs and the transition to NGPs, with global implications for inhaled medicines.
Erika Penz, Associate Professor of Respirology, Critical Care, and Sleep Medicine at the University of Saskatchewan, Canada, noted that suboptimal management of respiratory disease is associated with a disproportionately high burden on both patients and the environment. The forthcoming availability of pMDI medicines with NGPs alone will not resolve this larger issue. As every healthcare interaction carries a carbon footprint, which increases with the intensity of treatment, the implementation of guidelines into clinical practice would improve patient outcomes and reduce the demand on healthcare services and the associated carbon emissions.
Co-chair Helen Reddel, Clinical Professor and Research Leader at the Woolcock Institute of Medical Research, Australia, concluded by re-emphasising the urgent need to implement guidelines immediately for the benefit of both patients and the environment.
Respiratory Healthcare Delivery: Urgent Change Required
John Hurst
Healthcare decision-makers are facing significant challenges that will intensify in the coming years as they work to address health inequities, improve system resilience, and reduce carbon emissions. As Hurst highlighted, the global burden of respiratory disease continues unabated, placing a significant burden on patients, healthcare systems, and society. In the UK alone, 40% of asthma patients are uncontrolled,1 with delays in referrals to specialist services for severe asthma diagnoses and management.2 Additionally, COPD is a common cause of emergency admissions,3 with more than one in three patients not receiving maintenance therapy following one severe or more than one moderate COPD exacerbation(s).4 Only 44% of those who experience a COPD exacerbation survive after 5 years.5 Furthermore, Hurst reported a “lack of access to life-sustaining,” inhaled corticosteroid (ICS)-containing therapies in up to two-thirds of low-to-middle-income countries.6,7
The situation is expected to become ever more challenging as the global population ages, increasing the prevalence of respiratory diseases and the complexity of patients’ needs.8,9,10 These challenges are compounded by climate change, which poses a significant health risk and is associated with an increase in respiratory illnesses and other health emergencies.11,12 Hurst stated, “As respiratory clinicians, we have some big challenges on our hands, but this is not the only challenge, we have the wider challenge of planetary health, and as a subset, we should be thinking about climate change.” Rising temperatures are projected to cause an estimated 250,000 additional deaths annually between 2030–2050.12 Ironically, the healthcare sector itself is a major greenhouse gas (GHG) emitter; if it were a country, it would rank as the fifth-largest GHG emitter in the world.13 Therefore, healthcare is a critical sector for decarbonisation, and there is an urgent need to reduce carbon emissions now.
Hurst emphasised that these seemingly conflicting challenges of increasing demand and reducing carbon emissions do not need to be in conflict. He stated, “They are intimately connected, and by solving both together, we can achieve the most benefit.” Hurst advocated for urgent changes in the delivery model and for innovative solutions to provide respiratory care more efficiently and proactively by preventing disease onset, diagnosing disease early, and optimising its management.14-16 This approach would reduce the demand for urgent or emergency care and keep patients out of hospitals.14
To achieve a more preventative approach to respiratory care, Hurst proposed implementing global, evidence-based recommendations for COPD (e.g., the Global Initiative for Chronic Obstructive Lung Disease [GOLD])17 and asthma (e.g., the Global Initiative for Asthma [GINA]),18 into local guidelines and routine practice. He argued, “Implement these guidelines properly, and we improve the health of hundreds of millions of patients around the world, and at the same time improve planetary health.” This is crucial for optimising patient outcomes, reducing the strain on overstretched resources, and decreasing the carbon footprint of care.14-16,19 Hurst specified that while the delivery model for healthcare requires urgent change to achieve its goals, restricting clinical choice and access to innovative medicines is not the way forward.
The Importance of Inhaler Choice to Personalise and Optimise Treatment
Omar Usmani
Usmani expanded on Hurst’s message, highlighting that inhaled medicines are the foundation of treatment for patients with asthma and/or COPD.20 According to current guidelines, they are recommended for use across all stages of disease severity.17,18 It is therefore crucial to have a diverse range of inhalers and treatment regimens available to enable personalised and optimised care for respiratory diseases.17,18 The choice of an inhaled medicine should be guided by a combination of disease and patient-specific factors,21 which is why Usmani emphasised that inhalers should not be considered readily interchangeable.22
Considering the Disease, Drug, and Device for Personalised Respiratory Care
When selecting the most appropriate inhaler regimen, several factors must be considered for personalised care.17,18,23 Usmani summarised these factors as relating to the device, the drug, and the disease, emphasising that all three play a significant role in achieving the best possible patient outcomes. In terms of the disease, considerations include the diagnosis, severity, and presence of any comorbidities. The choice of the device relies on a patient’s ability for correct use, which is particularly important for vulnerable populations such as children, the elderly, or those with a compromised ability to use dry powder devices.24 To accommodate the heterogeneity of patients with respiratory disease, an array of inhaler delivery options are available, reflecting the principle that there is no “one size fits all” solution.24 Usmani emphasised, “We should personalise and select the best device for the patient.” Patient preference and satisfaction with their inhaler are also crucial to ensuring treatment adherence.21
Usmani further noted that what is often overlooked by healthcare professionals (HCP) is selecting the medication contained within the inhaler device. Multiple factors must be considered, including efficacy, safety, contraindications, dosing regimen, metabolism, and drug-to-drug interactions.25-28 He pointed out a common misconception: assuming that all drugs within the same pharmacological class have the same profile.25-28 To illustrate this, Usmani discussed the case of ICS, which are commonly prescribed either alone or in combination with bronchodilators for patients with asthma and/or COPD.17,18 Despite being in the same class, ICS can differ significantly in their pharmacokinetic and pharmacodynamic profiles leading to differences in their profiles for efficacy and safety, and the way they are recommended in guidelines.25-28
Usmani indicated that it is essential for HCPs to recognise and apply these fundamental elements to prescribe the right inhaler regimen for each patient. He stated, “We have a choice to personalise the drug and the device to our patients.” Thus, clinical choice is imperative to optimise patient outcomes.
Global Warming Potential of Current Inhaler Devices
The global warming (GWP) potential for current medical propellants has led some in the respiratory community to advocate for a blanket switch from pMDIs to DPIs.29 However, pMDIs play a critical role in respiratory care, representing 77% of inhaler usage globally and being the most commonly used inhaler type in 49 out of 53 countries analysed.30 Nevertheless, Usmani emphasised the importance of considering this in the context of total global anthropogenic carbon emissions, noting that the contribution of pMDIs is less than 0.04%.31
Usmani argued that implementing policies on the availability and use of pMDI medicines solely based on their environmental impact, without a patient-centric approach, could be detrimental to both patients and clinical practice.32 He also noted that in other areas of medicine with similar prognosis to asthma or COPD, patients are not denied effective treatments based on their carbon footprint. It is essential not to compromise on the quality of care by ensuring that the most effective medicines remain available.
Considering Patient and Healthcare Professional Perspectives in Inhaler Device Switching
Usmani highlighted the importance of ensuring that the voice of the respiratory community, including patients, is heard by those advocating for restrictions on inhaler device type, based on environmental factors. He presented recent findings from the largest global survey of its kind, which ranked the priorities of HCPs and patients with asthma and/or COPD when prescribing or choosing an inhaler device based on medical, non-medical, and environmental factors.33 The survey, conducted across 42 countries in six continents and involving 468 HCPs and 270 patients, found that clinical efficacy was ranked as the most important factor while environmental impact was considered the least important.33 Additionally, fewer than half (45.9%) of HCPs and less than two-thirds (60.1%) of patients expressed concern about the potential contribution of inhalers to climate change.33
Usmani also discussed the impact of non-clinical, non-consensual device switching and the potential for unintended consequences, such as unpredictable disease outcomes and increased healthcare resource utilisation (HCRU).34-36 He cited the potential for harm to the doctor-patient relationship especially when switches are without the patient’s consent.34,37 In qualitative surveys, patients have reported feeling a lack of control and believing their health and life were at risk in such situations.34
Moreover, a recently convened global expert panel on quality statements related to inhaler regimen switches concluded that it is essential to consider fully the time and resources required to implement such a switch, as well as the complexity of doing so at scale. The panel provided a practical checklist of essential activities to ensure any switch is both effective and safe.35
Approximately 18.2 million people with asthma and/or chronic lower respiratory disease (including COPD) use a pMDI as part of their care in the EU.35 Usmani described the significant challenges healthcare would face if environmentally driven policies enforced a blanket switch for a large number of patients. Data on the impact of switching inhaler regimens is limited; however, a systematic review of real-world outcomes concluded that it is difficult to predict clinical outcomes, including exacerbations and other factors that impact HCRU.34
Transition to Next-Generation Propellants (NGPs) for pMDI Inhalers
In the final part of Usmani’s presentation, he provided an update on the progress toward transitioning to NGPs, which have lower GWPs.38,39 His main focus was on hydrofluoroolefin (HFO)-1234ze(E) with a 100-year GWP of 1.4, which is considered a near-zero propellant.39 This is 99.9% lower than the propellant most commonly used today (hydrofluoroalkane (HFA)-134a).39 Using the example of a fixed-dose combination triple therapy pMDI approved for use in COPD, he showed that a device using HFO-1234ze(E) would reduce GHG emissions by 85%, bringing its carbon footprint in line with that of DPIs (Figure 1).40
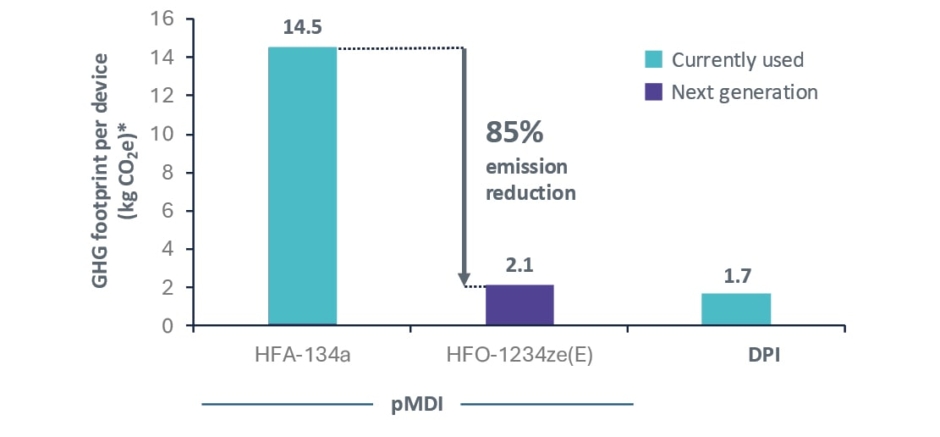
Figure 1: Next-generation propellant pressurised metered-dose inhalers containing HFO-1234ze have a similar carbon footprint to a dry powder inhaler.40
*Including prescription collection and delivery.
CO2e: carbon dioxide equivalent; DPI: dry powder inhaler; GHG: greenhouse gas; HFA: hydrofluoroalkane; HFO: hydrofluoroolefin; pMDI: pressurised metered-dose inhaler.
Usmani reported that the clinical trials required for regulatory approval of an HFO-1234ze(E)-containing NGP had been completed, with submission expected later in 2024 and availability anticipated from 2025.38 He emphasised that transition to NGPs is an important step in decarbonising healthcare, enabling the use of lower carbon devices without needing to change prescriptions or risk the potential for harm from non-clinically driven switches.
Usmani then highlighted draft legislation that is being evaluated by the ECHA for a wide restriction on PFAS chemicals which has global implications for inhaled medicines.41-44 The objective of the legislation is to minimise exposure to the environment of molecules which may be persistent. PFAS have been defined by chemical structure and the entire class of approximately 12,000 molecules is in the scope of the ban, including HFO-1234ze, despite a degradation half-life of 18 days.41,42 No exemption is currently proposed for medical propellants.45 He emphasised the importance of the respiratory community, as experts on the essential role of inhaled medicines, to inform the ongoing legislative process during the next public consultation phase expected in 2025. He provided a link for the audience to find more information on the ECHA proposal.46
The key takeaway from Usmani was that personalised inhaled therapy for those with asthma and/or COPD involves selecting both the medicine and the device.21 He emphasised that switching inhaler regimens for non-clinical reasons can lead to variable clinical outcomes.34 The transition to pMDIs with a carbon footprint similar to a DPI is expected to begin from 2025.38,40 Lastly, Usmani opined that the greenest inhaler is the one that a patient can and will use effectively,47 highlighting that the bigger challenge is addressing suboptimal care.
Addressing the Patient and Societal Burden of Suboptimal Respiratory Care and Its Environmental Impact
Erika Penz
The delivery of care is a contributor to healthcare GHG emissions, with more intensive treatment resulting in a higher carbon footprint.48 Penz highlighted that every healthcare interaction has a carbon footprint (Figure 2),49 which means that clinical choices can directly impact healthcare emissions and the carbon footprint of care.14 She used her presentation to illustrate how common suboptimal management of respiratory disease is associated with increased HCRU, from poor disease control, exacerbations, and increased use of short-acting β2-agonist (SABA) relievers, all of which contribute to increased GHG emissions.50 In addition to impacting patients and stretching healthcare resources, this also has environmental consequences.51 Thus, adopting a more preventative approach to managing respiratory disease would also offer an environmental benefit.
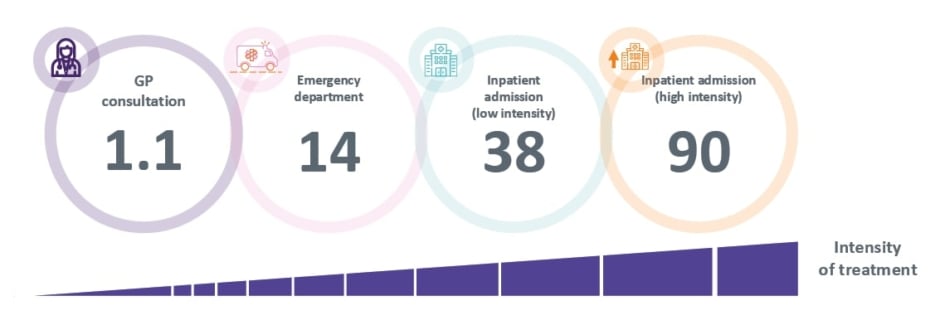
Figure 2: Greenhouse gas emissions (kg of CO2 equivalent) per healthcare resource utilisation event in the UK.49]
*Note this data excludes emissions associated to prescriptions.
GP: general practitioner.
Implications of Poor Respiratory Control on Carbon Footprint
According to the SABINA CARBON study, patients with poorly controlled asthma have a carbon footprint (measured as per capita [per 10,000 person-year] GHG emissions) approximately three times higher than those with well-controlled asthma.52 In the UK, the carbon footprint of overall asthma care is over 750,000 tonnes CO<sub>2</sub> equivalent (CO<sub>2</sub>e) per year.<sup>52</sup> Around 40% of this is attributed to treating the consequences of poor control, such as exacerbations and SABA overuse, amounting to approximately 300,000 tonnes CO2e per year.<sup>52</sup>
Overuse of SABA relievers is also common in COPD and is associated with poor clinical outcomes.<sup>53</sup> The high use of SABA is associated with a significantly higher risk of both moderate and severe exacerbations, as well as higher overall mortality rates.<sup>53</sup> Overall, SABA relievers constitute the majority of inhaler use and carbon footprint in Europe and Canada, ranging from 33–71% of inhaler use depending on the country,<sup>51</sup> contributing to two-thirds of the total GHG emissions from inhalers, and revealing that suboptimal treatment of respiratory disease remains widespread and has an environmental consequence.</up>51</sup>
Penz highlighted that the impact of even a single COPD exacerbation can extend beyond the lungs and last up to a year.54,55 The EXACOS-CV study found that the risk of severe cardiovascular events increases after even a moderate COPD exacerbation.54 More broadly, the SHERLOCK study showed that one or more moderate or severe exacerbations increased all-cause HCRU (comprising primary care, outpatient visits, and hospitalisations) in the 12 months of follow-up.55 Penz noted that the growing body of evidence suggests that, regardless of disease severity, taking a more preventative approach to COPD treatment is crucial for improving patient outcomes.
The consequences of suboptimal COPD care have a disproportionate impact on the carbon footprint of COPD-related HCRU. The carbon footprint of patients with COPD in England has recently been quantified in the EXACOS CARBON study.56 Notably, maintenance inhalers, which have been a focus of the inhaler device carbon debate and calls for switching, make a relatively minor contribution to the overall healthcare carbon footprint of these patients (63,867 tonnes CO2e for COPD maintenance inhalers alone), whereas the carbon footprint of COPD patients’ HCRU is approximately six-fold higher (414,187 tonnes CO2e of HCRU) than that of routine medicines.56 Penz also reported the disproportionate impact of COPD exacerbations on the carbon footprint. Patients who experienced two or more severe exacerbations had GHG emission rates seven-fold greater than those with no exacerbations (Figure 3).56 Overall, an estimated 6.8% of the GHG emissions of the National Health Service (NHS) in England result from the HCRU of patients with COPD.56
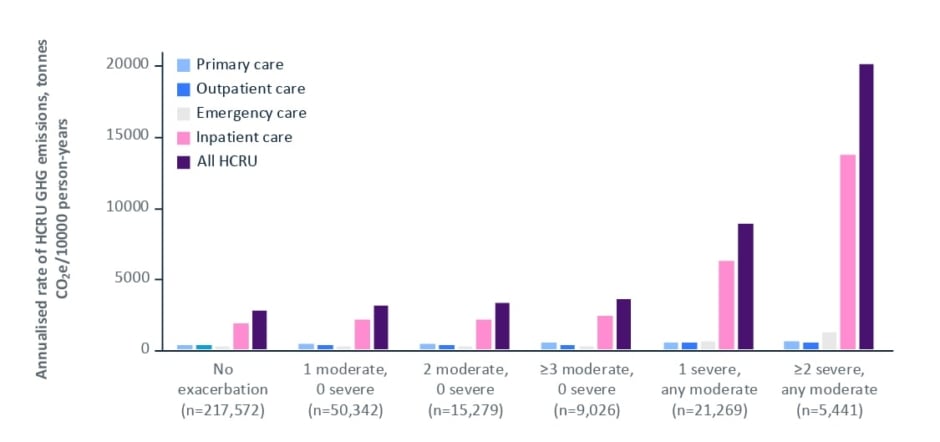
Figure 3: Greenhouse gas emissions from the HCRU of patients with COPD in England based on exacerbation history over a baseline period.56
CO2e: carbon dioxide equivalent; COPD: chronic obstructive pulmonary disease; GHG: greenhouse gas; HCRU: healthcare resource utilisation.
Real-World Impact of Guideline Implementation on Patient Outcomes and Carbon Footprint
Choices in patient care can directly impact the carbon footprint of healthcare.14 Penz stated that up-to-date, evidence-based treatment recommendations guide on how to optimise treatment for COPD17 and asthma.18 This was exemplified through several studies.
Regarding asthma care, the implementation of the GINA recommendations18 and the 2020 national New Zealand asthma guidelines (which prefer the use of an anti-inflammatory reliever) was associated with a reduced risk of hospital admission.57 Penz stated that the active implementation of the New Zealand guideline led to a >2-fold increase in the use of guideline-recommended therapy in the second half of 2022 compared with the second half of 2019.57 Over the 3 years following the implementation of the guidelines, there was a 17% reduction in hospital asthma discharges.57 These findings, Penz noted, underscored the importance of implementing guidelines to facilitate real-world practice changes that lead to improved patient outcomes.
Penz also summarised the SENTINEL programme, which involved a real-world, system-wide adoption of guideline-directed medical therapy in England, which included a maintenance and reliever therapy (MART)-preferred strategy; free from the use of SABA as a reliever as it does not treat the underlying inflammation in asthma.15,58 The quality improvement programme targeted those patients with asthma who were most at risk of an exacerbation, identified through high SABA prescribing (≥3 SABA canisters) or a history of exacerbations.15 A pharmacist-led treatment review was used to optimise their management in line with local guidelines, including the prescription of MART in appropriate patients.15 During the implementation phase, the proportion of patients prescribed MART increased approximately four-fold and was maintained over the observation period.15 The proportion of patients prescribed three or more SABA canisters over a 12-month period fell by 48.3%.15 Importantly, there was a 29.8% reduction in severe asthma exacerbations.15 SENTINEL is a quality improvement programme, that includes what is believed to be the first prospective analysis to investigate improvements in patient outcomes and carbon reduction through guideline implementation. The full results will be published in due course, but Penz reported interim data showing that SABA prescribing declined by 27,609 inhalers, equating to a saving of 773 tonnes of CO2e.58
Penz also highlighted that proactive and preventative care is crucial for COPD management. She presented real-world evidence from Canada’s Best Care COPD programme, which implemented a primary care-based, integrated disease management approach to improve patient outcomes in COPD.59 The programme, which involved collaboration between primary care providers, patients/caregivers, certified respiratory educators, and case managers, focused on education, skills, and treatment management.59 The trajectory of COPD-related and all-cause HCRU changed from an increasing trend over 3 years before the intervention to a sustained long-term reduction in both COPD-related emergency department visits and hospital admissions over the 3 years following implementation.59 Her message was that these choices in clinical care would also have a direct impact on its carbon footprint.
Penz also discussed PROMETHEUS Canada, a modelling study that investigated the potential impact of fully implementing the 2023 Canadian Thoracic Society COPD guideline recommendations for single inhaler triple therapy over a 10-year period in the country, which were new data being presented at the ERS conference.60 The study estimated that guideline implementation could decrease exacerbations (a 13.1% [n=200,000] reduction in ≥1 severe exacerbation[s] and a 17.5% [n=1,070,000] reduction in ≥1 moderate exacerbation[s]), decrease mortality (22.2% reduction in all-cause mortality, avoiding 180,000 deaths), and reduce HCRU and overall healthcare costs (7.7% reduction in severe exacerbation cost, and 12.6% reduction in moderate exacerbation cost), equating to total savings of approximately 3.5 billion CAD.60 As every healthcare interaction has a carbon footprint, the reduced demand on resources would also be expected to yield a carbon benefit.14
Penz concluded by re-emphasising that poorly controlled disease and exacerbations have a disproportionate impact on patients, healthcare systems, and the environment.51,52,55,56 Guideline recommendations indicate how to optimise treatment for both COPD17 and asthma.18 Therefore, implementing guidelines is a powerful way to improve patient outcomes and, consequently, reduce the carbon footprint of healthcare.15,58-60 This builds on Usmani’s perspective by saying that ‘the greenest patient is the one whose respiratory condition is well controlled’.47
Prioritising Patients and Planet: Advocating for Change in Respiratory Care
Helen Reddel
A more proactive, preventative model of healthcare is urgently needed to address the current and growing burden of respiratory diseases like asthma and COPD worldwide.14 Amid the increasing demand for healthcare and the need to address climate change, it is essential to maintain clinical choice and provide personalised treatment options for patients. Reddel suggested that this can be achieved by personalising respiratory care from the point of diagnosis and throughout its management. This includes selecting the right inhaled medication and device according to the individual’s needs, skills, and patient preferences.21
Reddel re-emphasised the importance of advocating for the implementation of guidelines to address suboptimal respiratory care and reduce environmental impact. Treatment recommendations such as GINA,18 highlight the importance of personalised treatment for optimal patient care. This approach includes ensuring access to the right class of medication, where optimal inhaler selection is the safest and most effective for both the patient and the planet, while also ensuring availability, patient satisfaction, and proper inhaler use, and taking into account their environmental impact.18 From 2025, the transition of pMDI propellants to more climate-friendly NGPs is expected to significantly reduce the carbon footprint of pMDIs to levels comparable to those of DPIs.38,40,61 However, Reddel highlighted that implementing guidelines now is also a powerful way to immediately improve outcomes for patients and reduce the carbon footprint associated with healthcare.15,58-60
Reddel then reminded the audience about the proposed restriction by the ECHA on PFAS chemicals. This proposal has global implications for inhaled medicines.43,44 Reddel urged her colleagues to share information about the ECHA proposal with their colleagues and to be aware of the potential unintended consequences on patient outcomes,37,43 as outlined earlier by Usmani. She also noted the upcoming opportunity to participate in the next round of consultation, expected in 2025, and provided a link for the audience to find more information about the ECHA proposal (available in the reference list).46
Conclusion
Healthcare is facing significant challenges due to increasing demand and limited resources, which are set to increase with an ageing population and the impacts of climate change. It is also a major contributor to GHG emissions,13 and the healthcare sector must strive to achieve near net-zero carbon emissions across societies within the next few decades. Suboptimal management of respiratory disease is common and is associated with a disproportionate burden on patients, healthcare services, and the environment. Every healthcare interaction has a carbon footprint. Transitioning to medicines with a lower carbon impact, as anticipated for pMDIs from 2025, is crucial to maintaining clinical choice and preventing the unintended consequences of inhaler switching. However, there is a more urgent need to advocate today for better implementation of respiratory guidelines to improve outcomes both for patients and the planet.







