Meeting Summary
The third Encuentro Latinoamericano de Infecciones Respiratorias Recurrentes (ELAIR) took place in Mexico City, Mexico, on 11th−12th May 2017. ELAIR brought together experts from across Latin America and further afield, continuing an extraordinary didactic exercise on the cutting-edge advances of respiratory medicine. Impressive progress has been made in the past 15 years, with new treatments available to manage and prevent airway infections. It remains to be seen how this might affect the related conditions of wheezing and asthma in predisposed and sensitised subjects. However, early data suggest that lower respiratory infection rates may reduce the development of the above conditions which are closely related to viral infections. Immunomodulators that both prime the immune system to fight infection and reduce inflammation are likely to play a major role in secondary and even potentially primary prevention of atopic diseases.
This article has a correction, made on 09.01.18.
The details of the correction are as follows: The word ‘Rational’ was edited to read ‘Rationale’ in the last paragraph heading on pg.17.
The prior version of the paper is available on request, please contact [email protected]
BASIC CONCEPTS IN IMMUNE SYSTEM FUNCTION
Immune System Development
Like other organ systems, such as the circulatory or respiratory system, the immune system is essential for human life. The organs of the immune system manage our interaction with the environment, forming protective barriers where necessary, and provide both active and passive defence against potential attackers.
The complexity of the human immune system reflects an evolutionary arms race of adaptation and counter adaptation which began up to 4 billion years ago with the simple biochemical defences of unicellular organisms. The key ability of self or non-self-recognition was developed in multicellular sponges and corals around 1.5 billion years ago; later, in prevertebrate coelomates present 600 million years ago, specialised cell cavities produced specifically adapted cells, among them immune cells capable of phagocytosis, a strategy used previously for feeding in unicellular organisms. In the same era, antimicrobial peptides (AMP) and pattern recognition receptions (PRR), which improved non-self-recognition, emerged. Four hundred million years ago, major histocompatibility complex (MHC) polymorphisms allowed a great leap forward in immune specificity, paving the road for adaptive immunity and the development of antigen-specific immunoglobulins (Ig) in reptiles. Finally, Ig diversified into role-specific subtypes, with the current Ig subtypes appearing in mammalian species approximately 200 million years ago.
The species-level evolution of the immune system driven by genetic changes is reflected in a microcosm by the ontogenic evolution of each person’s individual immunity. Ontogenic immune maturation occurs through epigenetic changes both in utero and throughout life. Indeed, even before fertilisation, sperm utilise potent immune-tolerising strategies to evade the female immune system and allow zygote formation; the mother’s immune system must likewise develop tolerance mechanisms to the alloantigens in the resulting zygote and fetus. During and after birth, the baby will be exposed to a host of antigens over a rapid period, the nature of which will affect immune-system maturation. Mode of birth, namely vaginal versus caesarean, changes the exposure to microbiota, thereby modifying the immune system; the immune system is also influenced by the baby’s developing microbiome which, in turn, has a strong influence on immune system development.1 In addition to the well-known direct effect of colostrum on immunity in the newborn, breastfeeding also modifies the baby’s microbiota. Breastfed babies have a more diverse gut microbiome, which in turn aids both immune development and homeostasis. This homeostatic effect of the gut microbiome continues throughout life, making maintenance of diversity and avoidance of deleterious factors, such as excessive antibiotic use, an important factor in maintaining a healthy immune system.
Epigenetic changes continue during childhood with the immune system reaching its functional peak in young adults. Following maturation, the major function-altering events in the immune system include the aforementioned reproductive immunotolerance and immunosenescence, the latter defined as the ageing process of the immune system. Immunosenescence increases the risk of infection and loss of control of inflammatory processes, which may predispose to heart disease, stroke, and Alzheimer’s disease, as well as some forms of neoplasia. In addition, autoimmunity increases with age, resulting in an additional risk of disorders such as rheumatoid arthritis, myasthenia gravis, and diabetes. To put this risk into context, the lifetime risks of cancer and autoimmune disease in developed countries are estimated at 30% and 10%, respectively.
Structure and Function of the Immune System
While infection control is a key immune system function, it is not its sole purpose; the repair of tissue and disposal of damaged or neoplastic cells are also fundamental roles of the immune system. Supporting the development of the gut microbiome, which in turn supports proper immune function, is a further crucial task. These disparate roles are interdependent, as illustrated by the repair of tissue following immune-related inflammation or the removal of cells infected by viruses, and the harmonious activity of these roles is central to proper immune function. Like many organs and systems, the overriding function of the immune system is adapting to maintain homeostasis in a constantly fluctuating environment. This adaptive imperative is likely to be the reason behind the conservation of the manifold immunological strategies developed during the evolutionary process.
Discussion of immunity often focusses on innate and adaptive responses, missing the equally important constitutive immunity. Constitutive immunity is stimulus-independent, consisting mainly of barriers, while innate and adaptive immunity are stimulus-dependent responses. The innate system is characterised by a fast but non-specific response, while the adaptive system acts more slowly but with a specific and more efficacious response that confers long-term immunity. Constitutive immunity includes epithelial barriers such as the skin, mucociliary transport, natural AMP, and antimicrobial enzymes like lysozyme. Innate immunity comprises innate lymphoid cells (ILC), complement, mast cells, neutrophils, eosinophils, and basophils. The major effectors of the adaptive immune system are the multiple T and B lymphocyte subtypes, the latter of which produce Ig. As with immune system functions, the classes of immunity are interdependent and overlapping. Innate and adaptive immunity must function in unison with different detector and effector cells working in concert and harmony, similar to musicians in a cellular symphony. Some cell types cross over, with dual roles in both modes of immunity.
Constitutive Immunity
The tight junctions between cells of the body’s various epithelial barriers protect against passage of viruses, bacteria, fungi, and allergens. The production of mucus by goblet cells and the co-ordinated movement of cilia facilitate the mucociliary transport system, further impeding the entry of pathogens and allergens and aiding in their expulsion. Sufficient humidity, correct temperature, and proper viscosity of the mucus are all key factors for the function of the mucociliary transport system.
AMP are anti-infective proteins produced at the constitutive barriers and are active against both Gram-positive and Gram-negative bacteria; in addition, AMP can attack the viral envelope, fungi, protozoa, and even cancer cells. AMP also have an immunomodulatory role, producing a chemotactic effect, helping to reduce inflammatory response and aid its resolution, and interacting with and moderating the adaptive immune response. AMP also help to regulate the microbiota and function by two principal mechanisms: the formation of transmembrane pores, which has a cytolytic effect, and the penetration of the cell membrane and disruption of essential processes through binding intracellular molecules.
Innate Immunity
Activation of PRR is a primary stage in initiating the innate immune response. There are several types of PRR; however, externally expressed toll-like receptors (TLR) are one of the most important types. TLR recognise products known as pathogen-associated molecular patterns (PAMP) and begin a rapid response. Following activation, internal signal transduction cascades involving MyD88 and Trif lead to proinflammatory cytokine release. These cytokines then activate neutrophils and macrophages which clear the pathogens.
Activated neutrophils will move to the pathogen via chemotactic mechanisms before adhering to, endocytosing, and digesting the pathogen via the process of phagocytosis. Neutrophils also combat infections through the release of antimicrobial compounds via a process known as degranulation. The final antimicrobial tactic of neutrophils is the release of neutrophil extracellular traps (NET). These NET comprise webs of DNA which contain antimicrobial products and trap microbes, forming a physical barrier, particularly in the blood. The NET can either be released while the neutrophil continues to function or upon cell death by lysis in a controlled form of apoptosis, known as NETosis. The NET released by the sacrifice of neutrophils not only trap pathogens and toxins, but also promote their disposal and tissue repair. In comparison, macrophages are simpler cells, depending on phagocytosis to trap micro-organisms before merging their prison-like phagosomes with lysosomes, creating phagolysosomes where the micro-organisms are digested. However, even these simpler cells have other metabolic roles within innate immunity.
Natural killer (NK) cells actively monitor cells, checking for a ubiquitous molecule alongside the MHC Class I molecule and killing all cells that lack this molecular ‘off’ switch. Both viral infection and neoplastic processes may result in the loss of MHC Class I. NK cells form the ILC-1 cell group, alongside ILC-2 cells that help control extracellular pathogens and ILC-3 cells that have roles in inflammation, antimicrobial defence against fungus and bacteria, and tissue homeostasis. The ILC lie close to epithelial barriers and appear to have a role in maintaining barrier function; dysfunction of ILC has been linked to allergic conditions associated with the epithelium, such as asthma or psoriasis.
ILC are phylogenically and functionally related to cells of the adaptive immune system, sharing the same precursor cells and roles in fighting infection. T helper (Th)2 cells are functionally related to the ILC-2 group, Th22 and Th17 are related to the ILC-3 group, and Th1 cells are related to the ILC-1 group, which also contains NK cells. The feature that differentiates the innate and adaptive cells is the expression of antigen-specific receptors by Th cell groups.
Adaptive Immune System
The two major classes of effector cell in the adaptive immune system are the T and B lymphocytes. T cells mount a relatively rapid specific cytotoxic response, while B cells proliferate and produce Ig to target infection. T cell activation begins with dendritic cells, a type of specialised macrophage that presents antigens alongside MHC Class II and costimulation molecules. The antigen presenting dendritic cells first phagocytose pathogens before coupling their antigens to MHC II. T cells, which recognise the antigen, are then activated, triggering an adaptive response.
Due to their antigen specificity, naïve T cells spend many hours moving within and between lymph nodes, scanning dendritic cells and searching for their antigenic ligand.2 Cytotoxic T cells activated by dendritic cells directly target virus-infected or neoplastic cells, while activated Th cells recruit and attract additional lymphocytes by releasing cytokines. The initial rapid effector T cell response, which confers immediate protection, is followed by a slower memory T cell response, which protects against attack by the same pathogen in the future.
Different types of Th cells release different cytokines and activate different downstream effector cells. Th1 cells recruit cytotoxic T cells, IgG-bearing B cells, and macrophages, principally defending against intracellular micro-organisms. In comparison, Th2 cells recruit eosinophils, mast cells, and B cells bearing IgG, IgA, and IgE, defending against extracellular pathogens, including parasites, and regulating the allergic response; the Th1 cytokine cascade inhibits Th2 activity and vice versa. Another important Th subtype is the Th17 cell, which plays a role in tissue inflammation, autoimmunity, and defence against extracellular pathogens.
Like the immune system, Th cells must remain in balance, and T regulatory (Treg) cells play an important role in maintaining this balance. When a specific immune response is mounted, one Th type may dominate the immune landscape to curb a particular form of infection, and Treg cells restore balance following resolution of the immune response. As well as controlling excessive activity, regulatory immune cells control the migration of effector cells from the lymphoid tissue to the mucosa, where defence against invading pathogens is finalised. Persistent imbalance in Th cell populations is associated with autoimmune disease.
B cells are the other major class of adaptive immune-effector cell, with a principal role of antibody production. The most common antibody response occurs through a T cell-dependent pathway, where B cells resident in the germinal centre of lymphoid follicles are activated by follicular Th cells. Activated B cells proliferate, often differentiating into plasma blasts that produce IgM for a rapid but weak affinity Ig response. The next step of the antibody response involves differentiation into long-lived plasma cells, rapid proliferation, and affinity maturation, which improves the affinity of the antibodies produced. At this stage, isotype switching of antibodies also occurs; for example, between IgM and IgG-type antibodies. Different Ig subtypes are functionally specialised; for example, IgE is specialised to defend against parasitic pathogens, while IgA acts at the mucosa where it is released and then binds to a secretory component allowing prolonged activity in the harsh proteolytic environment. Following the initial antibody response, memory B cells will be produced, conferring long-term immunity. It is important to note that the effector and memory stages of immunity are not limited to T and B lymphocytes. These stages are reflected in the regulatory cell classes that prevent excessive immune activity, with memory regulatory cells persisting long-term and awaiting activation by antigens alongside memory T and B cells.
Atopy and Allergy
Multiple predisposing factors result in an allergic response and genetic predisposition and epigenetic changes may both play a role. Diet, the microbiome, and environmental factors, such as pollution, are also likely to be involved. Dysfunction in the Treg environment may result in an imbalance in Th populations, which contribute to an allergic response. The genetic predisposition to produce IgE in response to common allergens is known as atopy. For allergy to occur, atopy must be combined with sensitisation by an allergen, which is then followed by hypersensitivity involving inadequate regulatory control of the response against the allergen. The final inflammatory stage of the allergic response is that recognised by patients as an allergic reaction, and involves the symptomatic presentation of allergic conditions such as atopic eczema, allergic rhinitis, and allergic asthma.
The Rational for the Use of Immunomodulatory Prophylaxis in Daily Clinical Practice
Respiratory tract infections (RTI) have become a clinical priority due to the substantial associated societal and personal burden of disease.3,4 Their effect is particularly grave in conjunction with serious chronic conditions, with over three quarters (78%) of exacerbations in chronic bronchitis and chronic obstructive pulmonary disease being due to RTI.5 Treatment of RTI is principally directed at symptom relief, using agents such as non-steroidal anti-inflammatory drugs to control fever and inflammation; however, antibiotics may be used to directly combat the infection when bacteria are the causal agents.
Viruses cause >80% of RTI; however, patient and family pressure can often cause physicians and pharmacists to prescribe antibiotics when evidence of bacterial infection is lacking.6,7 Such excessive use of antibiotics has contributed to antimicrobial resistance and, therefore, a global reduction in the use of antibiotics is an urgent priority. In this context, the use of prophylactic antibiotics should be limited to exceptional circumstances, such as in patients with primary immunodeficiency or failure of the immune system, for instance, in HIV-infected patients. Antimicrobial resistance makes the need for prophylactic strategies more pressing. Preventive measures against RTI include education, medical or surgical interventions, and both non-specific and specific immunomodulation.
Prevention of Respiratory Tract Infection
Behavioural interventions may be effective in reducing infection rates through a focus on parental education, encouragement of breastfeeding, and the reduction of modifiable risk factors, such as smoking in high-risk adult populations.8,9 Other modifiable risk factors that may be targeted in public health campaigns include exposure to pollutants, avoidance of overcrowding, and treatment of concomitant medical conditions.10-13
For certain pathological conditions, targeted medical interventions, such as vaccination, do have a role and are strongly recommended where available.14-16 Unfortunately, vaccinations are not available for the majority of pathogens responsible for common RTI.17 Surgical interventions, such as tonsillectomy, will likely continue to have a place in the prevention of specific recurrent infection, but this should continue to be a last-resort option.
Non-specific immunomodulation is a longstanding approach for the prevention of RTI and is now gaining increased prominence. The non-specific approach broadly reinforces the ability of the immune system to fight infection and various types of immunomodulators have been developed, including plant-derived substances, bacterial lysates, bacterial ribosomal preparations, thymic derivatives, synthetic peptides, and chemical substances.18 The quality of evidence for immunomodulators varies significantly; for example, despite use since the 1920s, evidence for the clinical benefit of Echinacea in treatment of rhinosinusitis has yet to be demonstrated.19 Data from the well-respected Cochrane collaboration indicates that immunomodulators based on extracts from bacteria that commonly cause RTI have the strongest evidence base.20-23 In addition, bacterial lysate immunomodulators have been used in Europe for >30 years.
BACTERIAL IMMUNOMODULATORS: MODE OF ACTION
Bacterial lysates contain PAMP and other antigens capable of activating the innate and adaptive immune system (Figure 1). M cells located in the gut mucosa transport antigens to specialised antigen presenting dendritic cells and macrophages; dendritic cell processes also infiltrate the mucosal barrier, allowing direct trapping of antigens, and TLR expressed by gut epithelial cells may also be activated following oral administration of bacterial lysates.24 As previously noted, dendritic cells are important for the activation of T lymphocytes, including Treg lymphocytes, which in turn activate B lymphocytes allowing the production of IgA, the key secretory Ig of the mucosal system (Figure 1).24 In addition, the improved mucosal activity of secretory IgA is derived from the secretory component that protects the antibody from degradation by proteolytic enzymes in the mucosa. Following detection of bacterial-lysate derived PAMP, the common mucosal immune system allows the spread of these gut-derived immune activities to lymph nodes in the mesentery and the chest, distributing antimicrobial activity to other mucosa.25
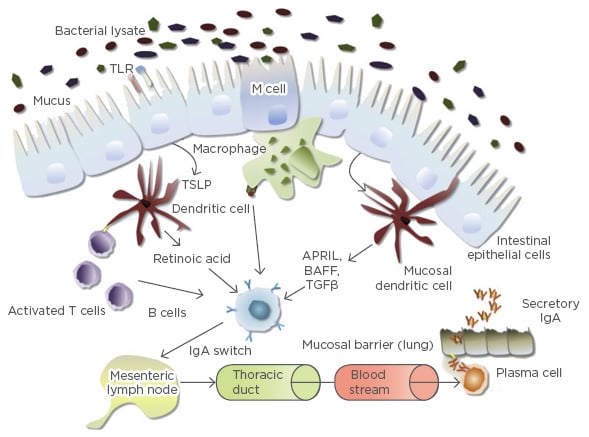
Figure 1: Immune system activation by oral bacterial lysates.
APRIL: a proliferation-inducing ligand; BAFF: B cell activating factor; IgA: immunoglobulin A; TGFβ: transforming growth factor beta; TLR: toll-like receptor; TSLP; thymic stromal lymphopoietin.
Adapted from Pfefferle et al.24
Immunomodulation by bacterial lysates involves both induction of immune system effector cells and activation of immunoregulatory cell classes. This effect mirrors that of the commensal microbiota, which both stimulate immune system maturation and reduce allergic sensitisation. Bacterial lysate immunomodulators induce immune-effector cells, reducing infection, and activate immunoregulatory cells, reducing inflammation. Evidence from in vitro, in vivo, and human trials indicates that the immunomodulatory effects of bacterial lysates induce effector cells in both the innate and adaptive immune system and their respective regulatory dendritic cell and regulatory T and B cell populations.26 Much of the immunomodulatory response is dependent on the previously mentioned TLR, expressed on epithelial cells, dendritic cells, macrophages, monocytes, and T and B lymphocytes. The type of TLR that is activated determines the downstream activity; production of cytokines raises an inflammatory response against pathogens, chemokines attract cells to the site of infection, and regulatory cytokines, such as interleukin (IL)-10, help to control inflammation.26
The innate response involves synergistic activation of TLR-4, TLR-2/6, TLR-9, and TLR-7/8. TLR-4 and 9 are the most extensively studied and evidence suggests that TLR-9 may be active in immunoregulation. Chemokines CXCL-1, CXCL-6, and CXCL-8 attract neutrophils that help fight bacteria and the activation of cytotoxic ILC-1 group cells improves both antibacterial and antiviral responses. Proinflammatory and antiviral cytokines induced by immunomodulators such as OM-85 as part of the innate response include IL-1β, IL-6, and tumour necrosis factor α, and IL-12, interferon (IFN)-α, IFN-β, IFN-γ, respectively.26
Bacterial lysate immunomodulators also induce cytokines related to the adaptive immune system including the B cell-activating cytokines IL-10, B cell activating factor (BAFF), and IL-6. Activation of the adaptive Th and B cell classes by immunomodulators has been demonstrated, as has the Ig response cascade of rapid IgM production, followed by IgG and IgA. Alongside IgA, which is central to the mucosal immune response, IgG has the advantage of being more widely distributed and providing a longer humoral memory response.26
The immunoregulatory effects of bacterial lysate immunomodulators involve maturation of both plasmacytoid and myeloid dendritic cells, indicated by the presence of T cell regulatory proteins CD80 and CD86. Activation of CCR7 has been demonstrated and is key for the migration of dendritic cells to mesenteric lymph nodes and bacterial-lysate related activation of CD4+ CD25+ FOXP3+ Treg cells has also been verified in vitro. There are several subpopulations of these regulators, like Treg 1, that predominantly produce IL-10, and Th3 cells that produce transforming growth factor beta (TGF-β). IL-10 is very important for the production of antibodies and for the control of inflammation, while TGF-β is necessary for lymphocyte maturation and IgA production. Regulatory cell expression of the chemokine receptor CCR9, which helps cells to migrate towards the mucosas, is also increased by immunomodulators.26,27
Mechanistic Data for the Bacterial Lysate Immunomodulator OM-85
OM-85 is the best-studied of the bacterial lysate immunomodulators.20 Data from Navarro et al.27 demonstrates activation of dendritic cells in the intestinal mucosa by OM-85 leading to antigen presentation and maturation of T cells to Th2 cells in a mouse model. These Th2 cells support B cells in the production of secretory IgA, before migration to the airway mucosa where they continue producing secretory IgA as well as TGF-β. Alongside the aforementioned functions, TGF-β aids tissue repair through activation of fibroblasts. The dual pro-immune/anti-inflammatory immunomodulator model is exemplified by the Type 1 regulatory T cell-related production of anti-inflammatory IL-10, induced by OM-85.27 This mouse data has been replicated in chronic obstructive pulmonary disease patients, where OM-85 induced a synergistic increase in the production of IL-10 under proinflammatory conditions.28 OM-85 also acts in a modulatory manner on molecular assemblages, known as inflammasomes, which are gaining increasing importance as key initiators of inflammation. Inflammasomes are sets of molecules that assemble inside the cell in response to infection, tissue damage, or metabolic imbalance, to activate the major inflammatory cytokines, IL-1β and IL-18, amongst others. Inflammasome assembly principally depends on three classes of molecules: a sensor molecule, an adaptor protein, and caspase 1. In the majority of currently described inflammasomes, the sensory molecules are nod-like receptors, a PRR related to TLR but located within the cytoplasm rather than on the cell surface. Nod-like receptors trigger inflammasome formation, where they assemble alongside adaptor proteins and pro-caspase 1; inflammasome formation results in pro-caspase 1 conversion to activated caspase-1. The subsequent protease activities of caspase-1 then result in fragmentation of the IL-1β and IL-18 precursors and their subsequent activation (Figure 2).
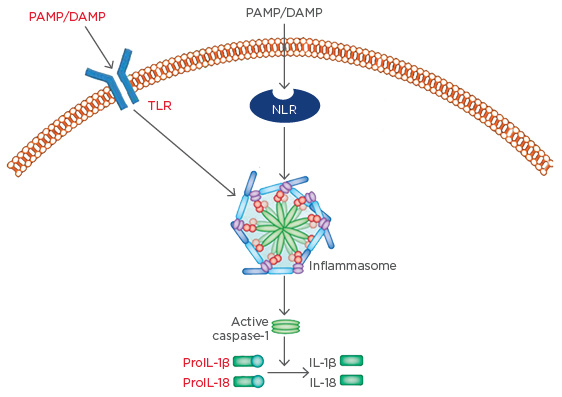
Figure 2: Inflammasome formation leads to the activation of inflammatory cytokines.
DAMP: damage-associated molecular patterns; IL: interleukin; PAMP: pathogen-associated molecular patterns; NLR: nod-like receptor; TLR: toll-like receptor.
Adapted from Shao et al.29
In a recent study, incubation of dendritic cells with OM-85 induced a pre-activated state in two inflammasomes important in viral airway disease, with no increase in pro-caspase levels. The authors suggested that this primed state may aid the inflammatory response following viral infection. In line with this hypothesis, production of antiviral IFN-β was also demonstrated in dendritic cells via OM-85-induced stimulation of the previously mentioned TLR Trif and MyD88. OM-85 also resulted in decreased release of inflammasome-dependent inflammatory cytokine IL-1β and reduced neutrophil, eosinophil, and macrophage activity, in a model of bacterial infection using challenge with lipopolysaccharide and the adjuvant alum.30
CONCLUSION
The functional immune system protects against infection while maintaining homeostasis in a fluctuating environment. Mechanistic evidence suggests bacterial lysate immunomodulators, such as OM-85, support immune homeostasis and help fight infections via the induction of an immune response, creation of a pre-alert inflammatory state, and a concurrent reduction in inflammation.








