Meeting Summary
This symposium took place during the 2024 European Respiratory Society (ERS) Congress held in Vienna, Austria. The main objective was to discuss the clinical aspects, diagnosis, and pathophysiology of bronchiectasis, a chronic, abnormal dilation of the bronchi, and its association with other lung diseases. The current understanding of the characteristics and prevalence of bronchiectasis in patients with chronic obstructive pulmonary disease (COPD) and alpha 1 antitrypsin (AAT) deficiency was discussed, as well as the relationship between the extent of traction bronchiectasis and exacerbations in idiopathic pulmonary fibrosis (IPF). The overarching message from the symposium was that advances are being made in elucidating the pathophysiology of bronchiectasis, and this is helping clinicians to understand why it occurs in patients with COPD and AAT deficiency. Increased characterisation of bronchiectasis is needed, including the understanding of its aetiology, disease development and progression, and the role of biomarkers in clinical management. This may help to identify treatable traits leading to personalised therapy with anti-inflammatory and antimicrobial drugs in the future.
Clinical Aspects and Diagnosis of Bronchiectasis
Bronchiectasis is a clinical condition defined as a chronic, abnormal dilation of the bronchi accompanied by classical symptoms.1,2 While the disease typically develops from chronic airway inflammation and/or infection, it has multiple aetiologies (excluding cystic fibrosis, which is considered a separate clinical entity) that can be associated with several different conditions (Figure 1).1,3
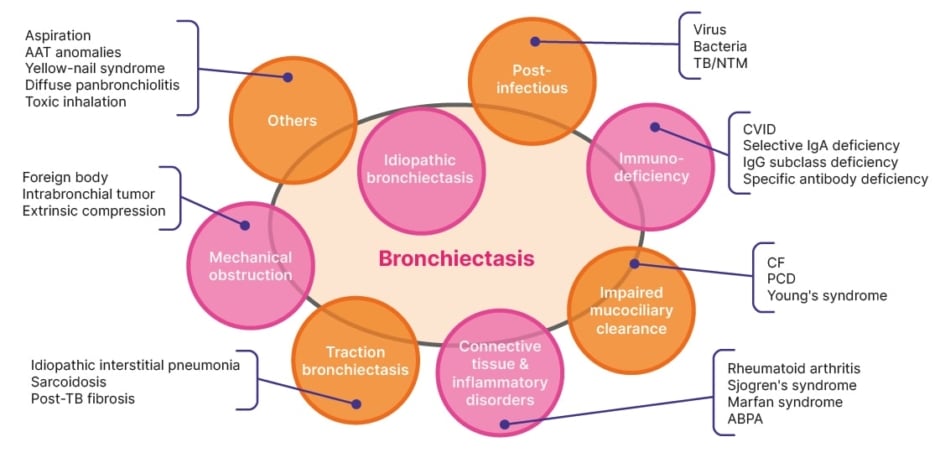
Figure 1: Major known causes of bronchiectasis.
Adapted from Chan ED et al. 2019.4
AAT: alpha 1 antitrypsin; ABPA: allergic bronchopulmonary aspergillosis; CF: cystic fibrosis; CVID: common variable immunodeficiency; NTM: non-tuberculous mycobacteria; PCD: primary ciliary dyskinesia; TB: tuberculosis.
International consensus recommendations indicate that the diagnosis of clinically significant bronchiectasis as a disease requires both radiological and clinical criteria, such as a persistent cough and sputum production, and a history of exacerbations.3 On CT, bronchiectasis appears as a dilation of the bronchus relative to the accompanying pulmonary artery, with a lack of tapering, bronchial wall thickening, and the presence of visible bronchi within 1 cm of the pleural surface.2
Franziska Trudzinski, a Senior Clinical Consultant at Heidelberg University, Germany, stressed that all patients with bronchiectasis should undergo an aetiological workup, which includes a review of their medical history, clinical findings, and radiological findings, and a “minimum bundle” of laboratory tests. The minimum bundle of aetiological tests recommended by the ERS for adults with a new diagnosis of bronchiectasis includes differential blood count to detect primary or secondary immunodeficiency, serum immunoglobins (total IgG, IgA, and IgM), and testing for allergic bronchopulmonary aspergillosis (ABPA).5 Trudzinski uses these three inexpensive tests, along with a test for AAT levels, to detect those causes of bronchiectasis that require a specific treatment in her patients, such as humoral immunodeficiencies or ABPA.
Patients also benefit from further examinations at a specialised centre if they are younger or have severe or rapidly progressing disease.5 For example:5,6
- sequential daily sputum cultures or a bronchoalveolar lavage should be considered if non-tuberculous mycobacteria are suspected;
- sweat chloride, other biomarkers, or genetic testing should be considered if cystic fibrosis is suspected;
- nasal nitric oxide, high-speed video analysis, transmission electron microscopy, immunofluorescence, and/or genetic testing should be considered if primary ciliary dyskinesia is suspected; and
- AAT serum levels, phenotyping, and/or genotyping should be considered if AAT deficiency is suspected.
Trudzinski emphasised that following the ERS recommendations can help to understand the aetiology of bronchiectasis, and can lead to relevant changes in treatment and prognosis.5 However, she explained that once bronchiectasis has been accurately diagnosed, additional CT scans are rarely useful unless the clinical manifestations of the disease have changed considerably; in patients with relatively stable disease, Trudzinski tends to perform a CT scan every five years (Trudzinski, personal communication).
She also stressed that a better understanding of the underlying pathology of bronchiectasis in different conditions is needed to enable clinicians to better tailor treatments to each patient’s disease rather than focussing purely on symptoms.
Bronchiectasis: State-of-the-Art
Eva Polverino, a Pulmonologist Expert in Respiratory Infections at the University Hospital Vall d’Hebron and VHIR, Barcelona, Spain, emphasised the heterogeneity of bronchiectasis. There are over 15 known causes of the disease (Figure 1); and bronchiectasis can be associated with other conditions such as COPD, rheumatoid arthritis, and severe asthma.7 In addition to cough and sputum production, clinical manifestations can include respiratory infections, lung function decline, and signs and symptoms of comorbidities.7 Accordingly, potential therapeutic options for the management of bronchiectasis vary and may include macrolides, pulmonary rehabilitation, long-acting bronchodilators, or inhaled corticosteroids (in patients with comorbid asthma).7
Bronchiectasis has long been understood to involve a ‘vicious cycle’ of host-mediated, inflammatory tissue damage and infection.8 However, Polverino explained that an infection of the respiratory tract is no longer considered to be the only trigger that can precipitate this cycle.
A more current view of the pathophysiology of bronchiectasis includes drivers such as airway inflammation, systemic inflammation, or genetic factors (Figure 2).7,9,10 Because the pathogenesis is more complex than was previously thought, Polverino stressed the need for a more personalised approach to treatment.
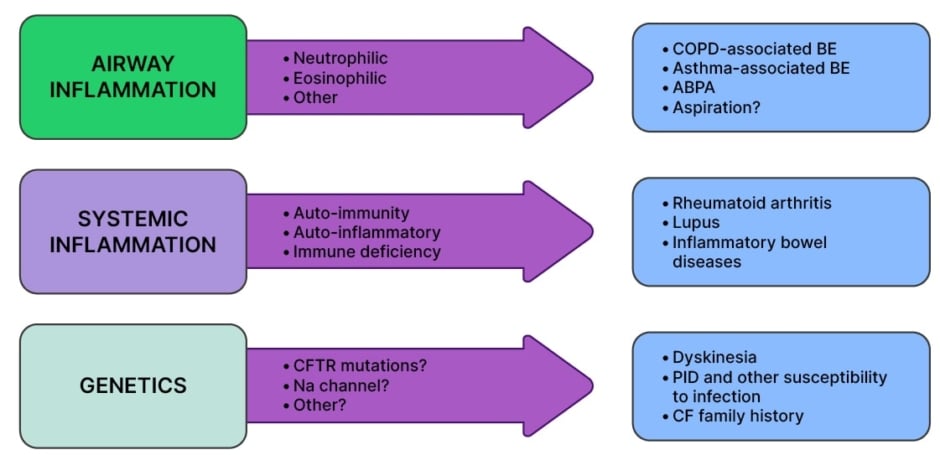
Figure 2: Pathophysiology of bronchiectasis.7,9,10
ABPA: allergic bronchopulmonary aspergillosis; BE: bronchiectasis; CF: cystic fibrosis; CFTR: cystic fibrosis transmembrane conductance regulator; COPD: chronic obstructive pulmonary disease; PID: primary immunodeficiency; PPM: potentially pathogenic microorganism.
Over a single year, half of patients with bronchiectasis in Europe experience two or more exacerbations, with approximately 25% of patients requiring at least one hospitalisation.11 Chronic infection in bronchiectasis involves immune dysregulation and is associated with a higher risk of exacerbations and hospitalisations, and reduced quality of life.12
The innate immune defence system carefully balances tissue repair, infection clearance, and injury resolution with the tissue damage that inflammation can cause. This balance requires the careful regulation of proteases (e.g., neutrophil elastase) and antiproteases (e.g., AAT).13 In bronchiectasis, dysregulation of this system tips the balance in favour of progressive tissue damage.13
In most cases of bronchiectasis, the disease is driven by neutrophilic inflammation,10,14 and a Phase II trial of an inhibitor of dipeptidyl peptidase 1, an enzyme that activates neutrophil proteases, resulted in fewer exacerbations and less sputum neutrophil elastase.15 Nevertheless, in about 20% of bronchiectasis patients eosinophil inflammation has been detected in the airways and has to be further addressed by future research to identify specific therapies.16
In addition to local inflammation, chronic respiratory disease can be associated with varying levels of systemic inflammation. Polverino explained that increasing evidence suggests systemic inflammation can be involved in bronchiectasis, and in some rare cases, this could be the driving factor in patients with rheumatoid arthritis or inflammatory bowel disease.7
Polverino stressed that real-world studies of bronchiectasis are incredibly important, particularly because of the diversity in patient demographics and healthcare systems. The European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) registry has generated considerable real-world data on the aetiological distribution of bronchiectasis. Huge differences have also been described in terms of local microbiology, for instance, in Southern Europe (Spain, etc.), there is a preponderance of Pseudomonas aeruginosa among patients with bronchiectasis, whereas Haemophilus influenzae is more common in Northern and Western Europe.11 EMBARC data has also estimated the prevalence of COPD and asthma among patients with bronchiectasis.17,18 Polverino emphasised that real-world data are invaluable for understanding the aetiology of the disease and determining how best to manage it in different geographical regions.
Polverino described a promising future for bronchiectasis management, with increased characterisation of the disease, through aetiology and biomarkers; personalised therapy with anti-inflammatory and antimicrobial drugs; and improved prevention through monitoring and immunisation, particularly in children at risk (Polverino, personal communication).
Bronchiectasis and Exacerbations in Chronic Obstructive Pulmonary Disease
There is a high prevalence of bronchiectasis among patients with COPD, although figures vary considerably between studies (4–72%).1 Marc Miravitlles, a Pulmonologist and Senior Researcher at the University Hospital Vall d’Hebron and Vall d’Hebron Research Institute (VHIR), Barcelona, Spain, explained that his own experience suggests the true prevalence of bronchiectasis is likely to be between 30–50% of patients with COPD. The presence of both conditions can be defined as the clinical COPD phenotype: COPD-bronchiectasis.1 However, he stressed the importance of differentiating the co-occurrence of bronchiectasis and COPD from bronchiectasis with airflow obstruction, which represents a purely bronchiectasis-based disease (Miravitlles, personal communication).
The European consensus definition of COPD-bronchiectasis, developed through a Delphi process by the EMBARC Airways Working Group, is the coexistence of four criteria, represented by the acronym, ‘ROSE’:19
- Radiology: abnormal bronchial dilatation, airways visible within 1 cm of pleura and/or lack of tapering sign in one or more pulmonary segment and in more than one lobe.
- Obstruction: a spirometry pattern of forced expiratory volume in 1 second/ forced expiratory volume <0.7.
- Symptoms: at least two characteristic symptoms from cough, expectoration, dyspnoea, fatigue, and frequent infections.
- Exposure: current or past exposure to smoke (≥10 pack-years) or other toxic agents (e.g., biomass).
Bronchiectasis in patients with COPD is usually cylindrical, bilateral, and basal, with moderate severity scores in radiological analysis.1 Patients with severe COPD are consistently more likely to have bronchiectasis than those with moderate COPD,1 and the probability is also higher in patients with potentially pathogenic microorganisms (PPM) isolated from sputum, and those with a greater number of hospital admissions in the previous year (Figure 3).20
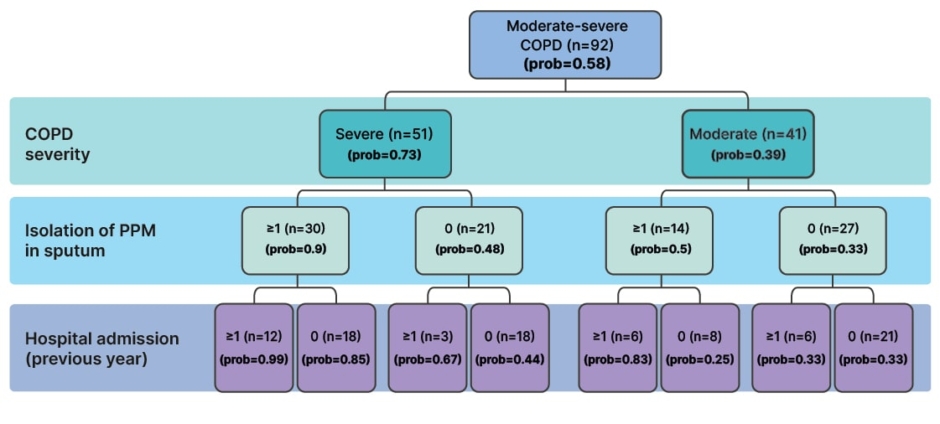
Figure 3: Probability of the presence of bronchiectasis by patient characteristics.
Adapted from Martínez-García et al. 2011.20
COPD: chronic obstructive pulmonary disease; PPM: potentially pathogenic microorganism; prob: probability.
Miravitlles explained that it is biologically plausible that COPD itself could cause bronchiectasis.1 Impaired immunity in COPD facilitates the survival and proliferation of PPMs in the lower airways, which can result in persistent bronchial inflammation. Together, chronic infection and inflammation can damage the bronchial wall and impair mucociliary clearance, leading to the vicious cycle of bronchiectasis.1 This evolution of COPD into a COPD-bronchiectasis phenotype could be driven by genetic predisposition, environmental factors, response to antibiotic treatment, and/or immune response.1 In Miravitlles’ opinion, bronchiectasis can develop as a consequence of COPD in those patients who experience frequent exacerbations, bacterial infection, and increased inflammation over the course of their COPD, due to the bronchial damage they sustain. On the other hand, Trudzinski explained that in patients who develop bronchiectasis in childhood and are then exposed to environmental triggers, COPD may develop as a secondary disease. (Trudzinski, personal communication).
The presence of bacteria in the lungs is associated with inflammation in a dose-response relationship; higher bacterial loads are associated with a higher intensity of inflammation and more frequent exacerbations.21 Miravitlles considers chronic bronchial infection to be the primary factor associated with both symptoms and poor outcomes in bronchiectasis, but he pointed out that frequent and severe exacerbations are also associated with a poor prognosis in COPD in general. He concluded that bacteria are likely be a foundation for the pathogenesis of bronchiectasis in COPD.
The best way to understand the natural history of bronchiectasis in COPD is by building large, international registries to collect information from a large population of patients. Miravitlles stressed that observational studies have helped to identify several factors associated with the development of bronchiectasis in COPD including: the frequency of severe exacerbations; the presence of bacteria in the lower airways, both during exacerbations and in the stable state; and the presence of purulent sputum.22-24 He explained that patients with these factors are at increased risk of developing bronchiectasis or of worsening existing bronchiectasis.
A prospective, observational, cohort study in Spain followed patients with moderate-to-severe COPD for around 8.5 years (102 months).22 Of the 77 patients who had at least two high-resolution CT scans for comparison, 16.9% had bronchiectasis that worsened, and 19.5% developed new bronchiectasis.22 Predictive factors for the progression or emergence of bronchiectasis in COPD included chronic mucopurulent/purulent sputum (adjusted hazard ratio [aHR]: 2.8; p=0.023), the number of PPM isolations (aHR: 1.1; p=0.011), and number of hospitalisations (aHR: 1.2; p=0.2).22 The presence of bronchiectasis in patients with COPD has also been associated with inflammatory cytokines in the sputum, poor lung function, exacerbations, and isolation of P. aeruginosa.23,24
Another prospective, observational study in patients with moderate-to-severe COPD found that bronchiectasis was associated with an increased risk of mortality in this population (HR: 2.54; p=0.02).25 The isolation of P. aeruginosa in patients with COPD is also linked with increased mortality (aHR: 1.95), and multiple isolates of P. aeruginosa have been associated with the presence of bronchiectasis and severe exacerbations.26,27
The sputum microbiome and protein profile in patients with COPD-bronchiectasis largely overlap with those in patients with bronchiectasis alone.28 However, compared with patients with COPD alone, those with COPD-bronchiectasis exhibit a greater abundance of proteobacteria, higher expression of mucin-5AC and proteins from the neutrophil degranulation pathway, lower expression of mucin-5B and peptidase inhibitors, and greater microbiome diversity.28
Understanding disease phenotypes in bronchiectasis is important because treatment strategies are based on the patient’s symptoms and risk factors.28 For example, Miravitlles considers frequent exacerbations to be the most important aspect of the disease due to the association with poor quality of life and reduced survival. In patients with frequent exacerbations, treatment strategies should be implemented to reduce their frequency and severity. Alternatively, if a patient is not a frequent exacerbator but has a chronic cough and sputum, or is short of breath, then treatment should aim to alleviate these symptoms.
Miravitlles summarised the hallmarks of bronchiectasis in patients with COPD as increased sputum production, recurrent infections, and frequent exacerbations. He stressed that a chest CT scan is recommended in these cases.29 The identification of treatable traits of COPD, such as AAT deficiency, chronic bronchial infection, and bronchiectasis, allows specific treatment to be tailored to individual patient needs.30
Same But Different? Bronchiectasis in AAT Deficiency
AAT deficiency is associated with unopposed protease activity, which drives an inflammatory cascade, resulting in enhanced airway inflammation. This represents a potential risk factor for the development of bronchiectasis.31-33
Despite this potential, Alice Turner, a Senior Clinical Lecturer in Respiratory Medicine at the University of Birmingham, UK, emphasised that the literature is unclear about whether an association exists between these two diseases.
Some studies have shown an increased prevalence of bronchiectasis in patients with AAT deficiency (9–27%) compared with the general population,31,33 and the prevalence of AAT deficiency among patients with bronchiectasis is similar to that observed for other genetic causes of bronchiectasis.33
However, prevalence estimates vary widely,34-36 bronchiectasis occurs at a similar frequency to usual COPD, suggesting that bronchiectasis is a secondary development,20,37 and there is no evidence of an allelic association.38
One reason for the lack of clarity regarding bronchiectasis in AAT deficiency is that several studies were of low quality, with heterogenous reporting, making it difficult to perform a meta-analysis.39
A recent analysis was conducted on data from the first 564 patients recruited to the European Alpha 1 Research Collaboration (EARCO) International Registry for whom a CT scan was performed.32 The data showed that bronchiectasis (with or without emphysema) was identified in 189 patients (34%), and 55 (9.8%) had bronchiectasis alone. However, it was also noted that forced expiratory volume in 1 second appeared to be impaired more by emphysema and smoking history than by bronchiectasis.32
Turner reported data from a recent study in 1,232 patients with AAT deficiency in Birmingham, UK.40 Among these patients, 235 (19.1%) had a diagnosis of bronchiectasis and 30 (2.4%) had a diagnosis of bronchiectasis without COPD. The prevalence of bronchiectasis was greater among patients with the severe deficiency PiZZ genotype of AAT deficiency than those with the milder PiSZ genotype (patients with PiZZ had significantly higher numbers of affected lobes; p=0.009).40 Even among those patients without COPD, bronchiectasis was still found to be associated with the PiZZ genotype (p=0.016), as well as with reduced serum AAT levels and poorer dyspnoea scores (Turner, personal communication).
However, no association was found between bronchiectasis and lung function decline measures or exacerbation rate,40 and Turner speculated that this may be because bronchiectasis is such a heterogeneous disease. Heterogeneity of bronchiectasis in AAT deficiency was shown by the broad range of individual patient scores in the Birmingham cohort using the validated Bronchiectasis Severity Index (BSI) scoring system.40
Mild bronchiectasis was predominant among those patients with AAT deficiency who were diagnosed with the disease, and bronchiectasis was even detected in CT scans from several of the patients in the control group who had not been diagnosed with bronchiectasis.40 Turner recommended that clinicians consider reviewing CT scans for patients with AAT deficiency to look for evidence of bronchiectasis.
In the Birmingham cohort, the majority (approximately 45%) of patients had cylindrical bronchiectasis morphology. Fifteen patients had the generally more severe cystic morphology, which was associated with a greater decline in the carbon monoxide transfer coefficient, and an increase in exacerbation rate compared with other morphologies.40
Of 994 sputum samples from patients with AAT deficiency, 61% showed evidence of bacterial colonisation, which is more common in patients with bronchiectasis (73%) than in those without (56%; Spittle, et al. Submitted for publication). The presence of colonising bacteria also appears to be associated with the rate of exacerbations.41 Studies to characterise the microbiome in AAT deficiency are ongoing, and it is expected that new data will help to define new phenotypes to explain the diversity of clinical presentations of lung disease in AAT deficiency.41,42
Turner concluded that, in some ways, bronchiectasis patients with AAT deficiency have similar characteristics to those in patients with COPD. However, the prognosis of patients with AAT deficiency with bronchiectasis is less clear, and there seems to be a greater heterogeneity of disease among these patients than is observed in patients with bronchiectasis alone.40
In terms of pathophysiology, early data suggest an association between AAT levels and bronchiectasis, and Turner explained that since bronchiectasis is also seen in patients without COPD, it is possible that reduced levels of AAT could be driving this pathology.43 In addition, polymers of AAT, often found in the circulation of patients with PiZZ AAT deficiency, can be considered pro-inflammatory and potentially chemotactic for neutrophils,44 and Turner speculated that these polymers could contribute to the vicious cycle of pathology in bronchiectasis.
Further radiological studies are needed to determine whether the morphological and microbiome data reported from the Birmingham AAT deficiency cohort can be replicated in other groups, and Turner stressed that we also know little about the drivers of exacerbations in AAT deficiency.
Bronchiectasis and Exacerbations in Idiopathic Pulmonary Fibrosis
On a CT scan, bronchiectasis with evidence of thickened, irregular bronchial walls (‘traction’ bronchiectasis) is an indicative feature of the usual interstitial pneumonia (UIP) pattern, which is typical of IPF, a progressive, life-threatening, interstitial lung disease of unknown aetiology.2,45,46 The rate of decline in patients with IPF can take different forms, including a slow physiologic deterioration, periods of relative stability interposed with periods of acute respiratory decline (exacerbations), or rapid deterioration.46 The occurrence of acute exacerbations in IPF is associated with a substantially increased risk of mortality.47
Luca Richeldi, Professor of Respiratory Medicine at the Catholic University of the Sacred Heart and Head of Pulmonology at the Gemelli Hospital in Rome, Italy, explained that the extent of traction bronchiectasis and/or honeycombing in CT may be a prognostic biomarker in fibrotic interstitial lung diseases (ILD) such as IPF.48 Among 209 patients with ILD, the risk of disease progression at 1 year was associated with both traction bronchiectasis and/or honeycombing (odds ratio: 8.54; p=0.19), and with raised levels of monocytes (odds ratio: 3.16; p=0.014).48 These three key variables commonly co-occurred in patients and were used to develop a validated scoring system for risk of progression in ILD: the honeycombing, traction bronchiectasis, and monocyte (HTM) score.48
In traction bronchiectasis, Richeldi stressed that inflammation is less relevant to disease compared to bronchiectasis in COPD. He explained that the role of inflammation remains controversial in IPF, and the main factor contributing to disease is fibrosis. For this reason, Richeldi feels that using purely inflammatory markers to monitor disease progression in IPF is unlikely to be clinically helpful.
Richeldi stressed that the treatment of acute exacerbations in IPF remains largely empirical and is not based on evidence.49 Currently, the best treatment for acute exacerbations is to avoid them by reducing disease progression.
Summary
The key messages among the emerging diagnostic and therapeutic concepts for inflammation in rare lung diseases discussed at this symposium were:
- The diagnosis of clinically significant bronchiectasis requires both clinical and radiological criteria, with further examinations at a specialised centre for younger patients, or those with severe or rapidly progressing disease.
- Many different underlying diseases are associated with bronchiectasis. The future is characterisation, personalised therapy, and addressing inflammation, infection, and prevention.
- Greater severity of COPD is associated with a higher prevalence of bronchiectasis, and the presence of bronchiectasis is associated with poorer prognosis.
- Bronchiectasis is not always reported on a CT scan for a patient with AAT deficiency. However, there is some evidence of an association between these two diseases, and a high rate of bacterial colonisation in the lung is associated with exacerbation risk.
- The risk of acute exacerbations in IPF correlates with the severity of the disease, i.e., the extension of honeycombing and traction bronchiectasis. The best treatment is currently to avoid acute exacerbations by reducing disease progression.
Trudzinski stressed that, currently, bronchiectasis is sometimes improperly reported, and that artificial intelligence is likely to be important in the future to better establish diagnostic criteria and markers of disease progression.







