Meeting Summary
Nebuliser therapy is a relevant therapy option for respiratory diseases, yet studies have demonstrated a wide variation in nebuliser drug delivery efficiency. This study assessed the aerosol performance of 15 commercially available nebuliser systems based on a European standard, and calculated the respirable drug delivery rate (RDDR) as an objective, clinically important measure of efficiency. Findings confirmed that the efficiency of nebuliser systems differs significantly, which could potentially impact the therapeutic success of the drug delivered. The authors of the study recommend that physicians select a device with a high RDDR to ensure that patients receive clinically effective doses in a short time and thus achieve the best possible treatment effect.
Introduction
For physicians, it can be a challenge to identify the performance differences between the many nebuliser systems available on the market. Nebuliser therapy is a relevant therapy option for respiratory diseases. Studies have demonstrated a wide variation in nebuliser drug delivery efficiency.1,2 Ralf Fischer, Medical Affairs Manager at PARI GmbH, Starnberg, Germany, stressed that sufficient and targeted drug deposition appropriate to the underlying lung disease, severity stage, and patient population is the crucial factor for clinically successful nebuliser therapy. Consequently, National Institute for Health and Care Excellence (NICE) guidelines recommend the use of a nebuliser system that is “known to be efficient.”3
Pulmonary drug deposition is significantly influenced by aerosol characteristics, airway anatomy, and breathing pattern of the patient.1 Of these three factors, the choice of an efficient nebuliser is a rapidly effective way for a medical practitioner to optimise aerosol delivery.1 Today, clinicians can select from a vast range of jet nebuliser systems, but there are limited objective comparison data available to help them make this choice.
The current European standard for nebuliser systems (EN ISO 27427) recommends that aerosol output (AO), aerosol output rate (AOR), and mass median aerodynamic diameter (MMAD) are reported by manufacturers, to allow for comparisons to be made between systems.2 However, these values are of limited relevance from a clinical point of view. For example, a high AO does not necessarily mean a high amount of drug in the lungs. Only the combination of AO with respirable fraction (RF; representing the proportion of particles <5 µm) to calculate the respirable dose (RD) indicates how much therapeutically effective aerosol potentially reaches the lungs. RD can be calculated as RD=RF×AO.2 Fischer emphasised that nebuliser efficiency depends not only on RD, but also on the rate at which the RD is delivered. Thus, the respirable drug delivery rate (RDDR=RF×AOR) represents an objective and clinically more relevant parameter for nebuliser efficiency (Figure 1).
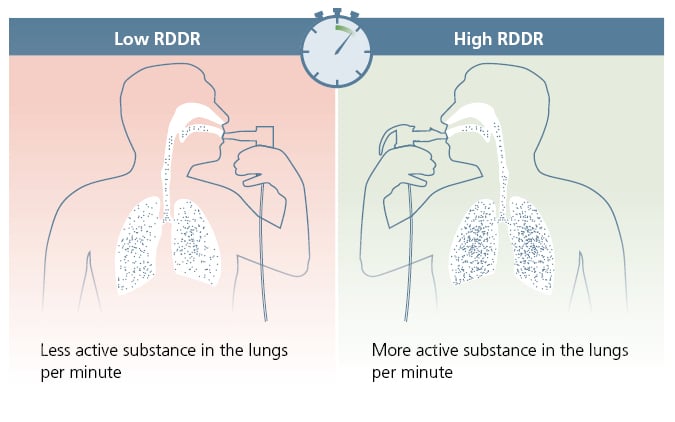
Figure 1: Respirable drug delivery rate.
Reproduced with permission from PARI GmbH. R
DDR: respirable drug delivery rate.
For these reasons, a study was conducted to assess aerosol performance of commercially available nebuliser systems according to the EN ISO 27427, which provides a benchmark comparison of aerosol performance on a standardised basis. From these data, the RDDR was calculated as an objective measure of efficiency.
Methods
Fifteen types of jet nebuliser systems were evaluated. For each type of nebuliser, AO, AOR, MMAD, and RF of three individual systems were measured in duplicate, providing a total of six readings.
Nebulisers were filled with 2 mL of 0.1% (w/v) salbutamol. Salbutamol amount was determined by a validated ultraviolet detector-tagged high-performance liquid chromatography system. AO, AOR, and nebulisation time were measured with a PARI COMPAS breath simulator (PARI GmbH, Starnberg, Germany) using a tidal volume of 500 mL, 15 breaths/min, with an inhalation:exhalation ratio of 50:50. MMAD and RF were measured with a cooled (17 °C) Next Generation Impactor (Copley Scientific, Nottingham, UK) at 50% relative humidity and 23 °C ambient conditions, at a flow rate of 15 L/min. RD was calculated as the product of AO and RF. RDDR was calculated as the product of AOR and RF.
Significance comparison of the mean RDDR was assessed using analysis of variance and Fisher pairwise comparisons, with a significance level of p<0.05.
Results
The systems with the highest AO were the Omron C28P/Omron X105 (OMRON Healthcare, Kyoto, Japan), PARI COMPACT2 (PARI GmbH), and MPV MicroDrop® Family2 (MVP Medical GmbH, Munich, Germany; 530 μL, 520 μL, and 520 μL salbutamol, respectively). The AO of these systems was almost three-times higher than the system with the lowest AO, the aponorm® Compact PLUS (Microlife, Widnau, Switzerland) on the maximum setting (190 μL; Figure 2).
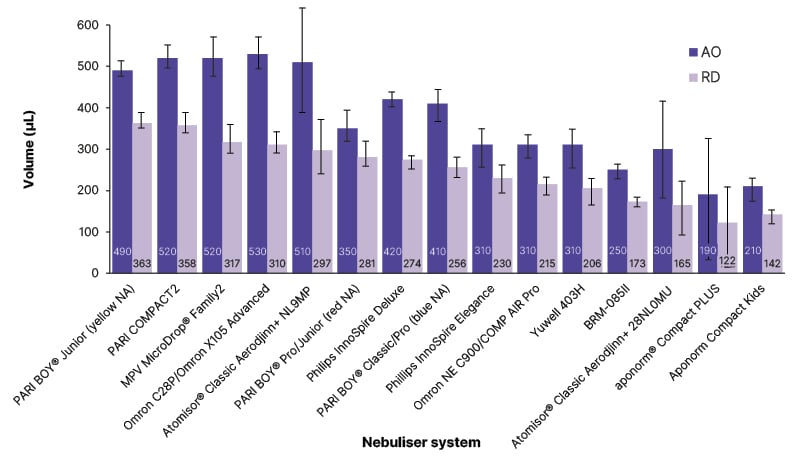
Figure 2: Mean aerosol output and respirable dose across 15 jet nebuliser types.
Error bars indicate standard deviation.
Reproduced with permission from PARI GmbH.
aponorm® Compact Kids: Microlife, Widnau, Switzerland; aponorm® Compact PLUS: Microlife; Atomisor® Classic Aerodjinn+ 28NL0MU: DTF Medical, Saint-Etienne, France; Atomisor® Classic Aerodjinn+ NL9MP: DTF Medical; BRM-085II: Bairui Medicine Co, Guangzhou, China; MVP MicroDrop® Family2: MVP Medical GmbH, Munich, Germany; Omron C28P/OMRON X105 Advanced: OMRON Healthcare, Kyoto, Japan; Omron NE C900/COMP AIR Pro: OMRON Healthcare; PARI BOY® Classic/Pro (blue NA): PARI GmbH; PARI BOY® Junior (yellow NA): PARI GmbH; PARI BOY® Pro/Junior (red NA): PARI GmbH; PARI COMPACT2: PARI GmbH; Philips InnoSpire Deluxe: Philips, Amsterdam, the Netherlands; Philips InnoSpire Elegance: Philips; Yuwell 403H: Yuwell, Shanghai, China.
AO: aerosol output; NA: nozzle attachment; RD: respirable dose.
In terms of the more clinically relevant parameter, RD, the PARI BOY® Junior (yellow nozzle attachment [NA]; PARI GmbH), PARI COMPACT2 (PARI GmbH), and MPV MicroDrop® Family2 (MVP Medical GmbH) had the highest RD values among the evaluated nebuliser systems (363 μL, 358 μL, and 317 μL, respectively).
The AO and RD data showed that a nebuliser system with a high AO does not necessarily deliver a high amount of RD. However, RD is a crucial parameter for the success of nebuliser therapy.
The RDDR, which considers the duration of nebulisation, also varied considerably. The nebuliser system with the highest RDDR displayed a value approximately three-fold higher than the system with the lowest RDDR. The PARI BOY® Junior (yellow NA; PARI GmbH), the PARI COMPACT2 (PARI GmbH), and the PARI BOY® Classic/Pro (blue NA; PARI GmbH) showed the highest RDDR values (115 μL/min, 105 μL/min, and 99 μ/min, respectively), which differed significantly (p<0.05) from all other nebulisers tested (Figure 3).
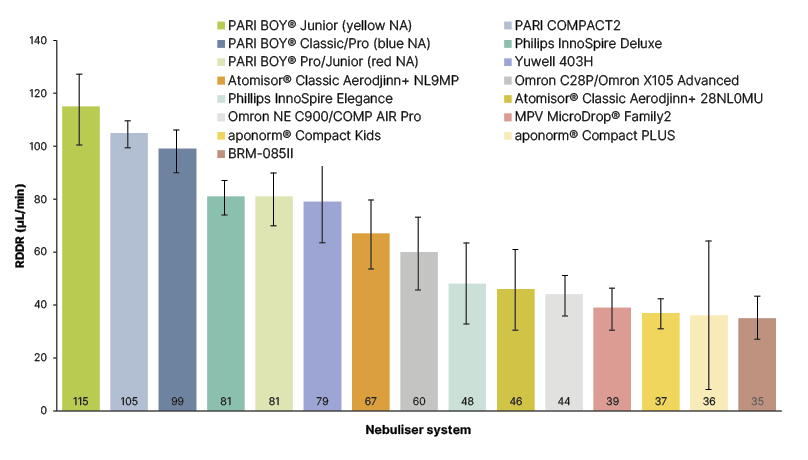
Figure 3: Mean respirable drug delivery rate across 15 jet nebuliser types.
Error bars indicate 95% confidence interval.
Reproduced with permission from PARI GmbH.
aponorm® Compact Kids: Microlife, Widnau, Switzerland; aponorm® Compact PLUS: Microlife; Atomisor® Classic Aerodjinn+ 28NL0MU: DTF Medical, Saint-Etienne, France; Atomisor® Classic Aerodjinn+ NL9MP: DTF Medical; BRM-085II: Bairui Medicine Co, Guangzhou, China; MVP MicroDrop® Family2: MVP Medical GmbH, Munich, Germany; Omron C28P/OMRON X105 Advanced: OMRON Healthcare, Kyoto, Japan; Omron NE C900/COMP AIR Pro: OMRON Healthcare; PARI BOY® Classic/Pro (blue NA): PARI GmbH; PARI BOY® Junior (yellow NA): PARI GmbH; PARI BOY® Pro/Junior (red NA): PARI GmbH; PARI COMPACT2: PARI GmbH; Philips InnoSpire Deluxe: Philips, Amsterdam, the Netherlands; Philips InnoSpire Elegance: Philips; Yuwell 403H: Yuwell, Shanghai, China.
NA: nozzle attachment; RDDR: respirable drug delivery rate.
Conclusions
Findings from this study confirmed that the efficiency of commercially available nebuliser systems differs significantly. Fischer pointed out that these performance variations could potentially impact the therapeutic success of the drug delivered through the nebuliser, by underdosing or delayed symptom relief. In the case of nebulised antibiotics, for example, insufficient nebuliser efficiency has been implicated in the development of resistance. In vitro studies found that less efficient nebulisers did not achieve the minimal inhibitory concentrations of antibiotics sufficient to eradicate a target organism.4
Fischer concluded that the RDDR represents an objective parameter for the efficiency of a nebuliser system. He emphasised that physicians should select a device with a high RDDR to ensure that patients receive clinically effective doses in a short time, and thus achieve the best possible therapeutic effect.
041D0719







