Presenters: Michael E. Wechsler,1 Nicola A. Hanania,2 Jorge F. Maspero3
1. National Jewish Health, Denver, Colorado, USA
2. Baylor College of Medicine, Houston, Texas, USA
3. Fundación Cidea, Buenos Aires, Argentina
Disclosure: Dr Wechsler has received grants and personal fees from GlaxoSmithKline and Sanofi; and personal fees from AstraZeneca, Boehringer Ingelheim, Equillium, Gala Therapeutics, Genentech, Genzyme, Mylan, Novartis, Pulmatrix, resTORbio, Regeneron Pharmaceuticals, Sentien Biotechnologies, and Teva. Dr Hanania has received research support and consultancy fees from AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Novartis, and Sanofi; research support from Gossamer Bio; and consultancy fees from Regeneron Pharmaceuticals. Dr Maspero has received consultancy fees from AstraZeneca, Sanofi, and Teva; speaker fees from GlaxoSmithKline, Menarini, Novartis, and Uriach; and research grants from Novartis.
Acknowledgements: Writing assistance was provided by Dr Samantha Stanbury, Stockport, UK.
Support: The publication of this article was sponsored by Sanofi Genzyme Europe B.V. Sanofi Genzyme and Regeneron are committed to providing resources to advance research in respiratory medicine in areas of unmet medical needs among patients with poorly controlled asthma and nasal polyps.
Citation: EMJ Respir. 2020;8[1]:50-56.
Meeting Summary
Dupilumab (Dupixent®) is a fully human monoclonal antibody1 that inhibits IL signalling of both IL-4 and IL-13, which are drivers of type 2 inflammation in multiple atopic diseases, including asthma.2 Dupilumab has been developed as an add-on maintenance treatment for asthma in adult and adolescent patients. There are some differences in specific indications of disease severity and clinical characteristics on the USA and European Union (EU) labels,3,4 and in the EU the approved indication for dupilumab in asthma is specifically for patients exhibiting type 2 inflammation, characterised by elevated blood eosinophils and/or raised fractional exhaled nitric oxide (FeNO).4 The clinical development programme for dupilumab in asthma (LIBERTY ASTHMA) includes the pivotal Phase III trials QUEST5,6 and VENTURE,7,8 and an open-label extension study (OLE), TRAVERSE,9 which recruited patients from QUEST, VENTURE, and two Phase II studies.
Dr Wechsler presented the first long-term efficacy and safety data to emerge from TRAVERSE at the European Respiratory Society (ERS) International Congress 2020. He showed that the long-term safety profile of dupilumab was consistent with that observed in the parent studies and that efficacy was maintained, including a persistently low exacerbation rate and improvements in lung function sustained for up to 96 weeks. Dr Hanania presented a poster reporting a post hoc analysis of QUEST, which explored exacerbation rates in patient subgroups defined according to degree of lung function improvement. Dr Maspero presented a similar post hoc analysis of VENTURE, which was a study in patients who were glucocorticoid-dependent, exploring relationships between oral corticosteroid (OCS) use and lung function improvement. Both analyses showed that dupilumab improved outcomes (exacerbation rates and OCS use, respectively) across patient subgroups with varying degrees of improvement in lung function.
Dupilumab Long-Term Safety and Efficacy in Patients with Asthma: LIBERTY ASTHMA TRAVERSE
Doctor Michael E. Wechsler
LIBERTY ASTHMA TRAVERSE,9 the first long-term study of the safety and efficacy of dupilumab in asthma, was an OLE study in patients with asthma who had participated in any of four Phase II or III clinical trials of dupilumab: a Phase IIa proof-of-concept study (EXPEDITION),10 a Phase IIb dose-ranging investigation,11 and the Phase III QUEST6 and VENTURE8 studies. Patients entering TRAVERSE received open-label treatment with dupilumab 300 mg every 2 weeks (q2w) for 48–96 weeks. The study population comprised 2,282 adult and adolescent patients (aged ≥12 years) with moderate-to-severe asthma, including some patients with OCS-dependent asthma.
Dr Wechsler presented the headline safety and efficacy results from TRAVERSE. The safety profile was consistent with that seen in the parent studies, and no new safety signals were observed. Treatment-emergent adverse events were experienced by 76–88% of patients at any time during TRAVERSE, across study groups based on parent study and randomised treatment group in the parent study. The most common treatment-emergent adverse events were nasopharyngitis and injection site erythema. The rate of serious adverse events was 9–13%.
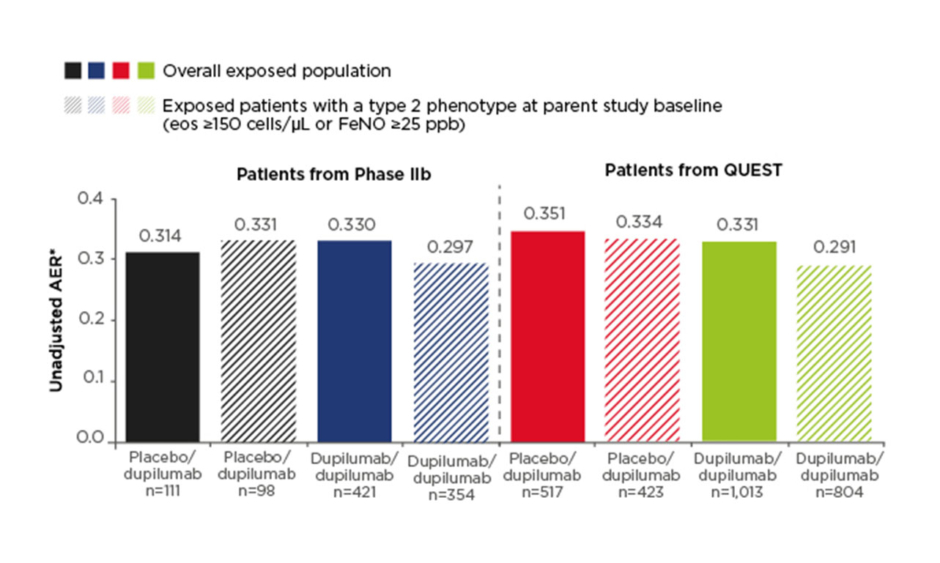
Efficacy results were presented for the non-OCS-dependent patient population, which included patients recruited from the Phase IIb study and the Phase III QUEST study (n=2,062). The primary efficacy endpoint was annualised exacerbation rate (AER). Low exacerbation rates observed in the parent studies were sustained in the OLE and ranged from 0.31 to 0.35 across study groups in the overall exposed population (Figure 1). This compares with baseline AER of 1.85–2.37 across treatment groups at the start of the Phase IIb and QUEST studies. A subgroup of patients with type 2 asthma phenotype (defined as eosinophils ≥150 cells/µl or FeNO ≥25 parts per billion at parent study baseline; n=1,679) had similar results, with AER during the TRAVERSE study ranging from 0.29 to 0.33 (Figure 1).
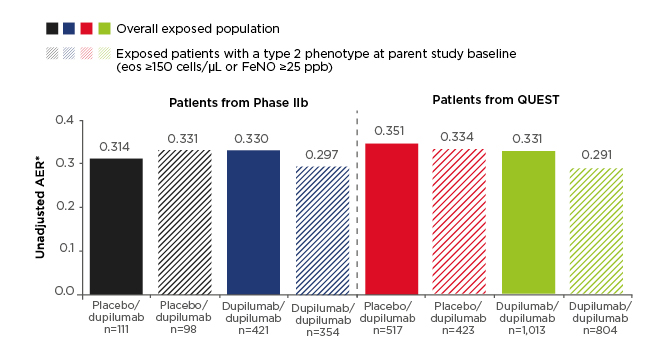
Figure 1: Annualised exacerbation rate in the non-oral-corticosteroid-dependent population in LIBERTY ASTHMA TRAVERSE.
AER was assessed in the exposed population (observed cases).
*The total number of events that occurred during the treatment period divided by the total number of patient years in the treatment period.
AER: annualised exacerbation rate; eos: eosinophils; FeNO: fractional exhaled nitric oxide; ppb: parts per billion.
An analysis of lung function based on forced expiratory volume in 1 second (FEV1) showed that for patients who were randomised to dupilumab in the parent studies (Phase IIb or QUEST), improvements in FEV1 were sustained throughout the OLE, while patients who received placebo in the parent studies had rapid improvements in FEV1 on commencing dupilumab in the OLE. Baseline FEV1 was 1.75–1.86 L across treatment groups at baseline in the Phase IIb and QUEST parent studies, increasing to 2.02–2.12 L by Week 96 of TRAVERSE, representing a 13–22% improvement in the non-OCS-dependent population. Similar improvements in lung function, by 13–25%, were seen in the subgroup of patients with type 2 phenotype asthma.
Transient increases in eosinophils have been observed in dupilumab clinical studies, including parent studies feeding into TRAVERSE, and are consistent with the mechanism of action of dupilumab.5,7,12 In the parent studies, mean eosinophil counts returned to baseline or close to baseline by the end of the study.5,7,12 Monitoring of eosinophil counts continued in the OLE for patients in the non-OCS-dependent population (patients recruited from the Phase IIb and QUEST studies). Transient increases were observed during the first few weeks of TRAVERSE in patients who had received placebo in QUEST and commenced dupilumab treatment on entering TRAVERSE, and in patients entering TRAVERSE from the Phase IIb study, who had a 16-week treatment-free follow-up period between completing the double-blind treatment period in the parent study and entering the OLE; these increases resolved by Weeks 12–24. Longer-term follow up showed that, by Week 96 of the OLE, mean eosinophil counts had decreased to below parent study baseline levels.
Eosinophils are a key marker of type 2 asthma. Serum IgE is an additional marker, specifically of allergic asthma, which is characterised by IgE-mediated type 2 inflammation.13 Serum IgE has been measured as an exploratory biomarker in dupilumab asthma studies. Data on serum IgE were available for patients who participated in the Phase IIb study, and serum IgE measurements were repeated in these patients (n=532) during the OLE. At the start of the OLE, mean serum IgE was lower in patients from the dupilumab treatment group compared with those from the placebo group in the Phase IIb feeder study. By Week 96 of the OLE, mean IgE levels dropped markedly in both groups, decreasing by 82% from parent study baseline in the overall Phase IIb study population.
Dr Wechsler concluded that long-term treatment with dupilumab was generally well tolerated, and that the clinical efficacy observed in the parent studies was maintained, including a persistently low exacerbation rate and sustained improvements in lung function. Markers of type 2 inflammation also decreased during long-term dupilumab treatment.
Effect of Dupilumab on Severe Exacerbations in Asthma Patients With and Without Lung Function Improvements
Doctor Nicola A. Hanania
LIBERTY ASTHMA QUEST study is the largest randomised controlled trial of dupilumab in patients with asthma, contributing >50% of the patients who enrolled in the TRAVERSE study described above. In the QUEST study, a total of 1,902 adult or adolescent patients (aged ≥12 years) with uncontrolled moderate-to-severe asthma were randomised to treatment with dupilumab 200 mg or 300 mg q2w, or matched placebo, for 52 weeks. The primary endpoints were annualised rate of severe exacerbations over 52 weeks and change from baseline to Week 12 in prebronchodilator FEV1.5 Significantly lower annualised rates of severe exacerbations were recorded in both dupilumab groups compared with matched placebo (46–48% relative reductions), while mean increases in FEV1 were significantly greater in dupilumab groups (320–340 mL) compared with placebo (180–210 mL).5
Dr Hanania presented a post hoc analysis exploring the relationship between these two outcomes. The objective of the analysis was to assess the efficacy of dupilumab in reducing severe exacerbations in subgroups of patients who did and did not achieve clinically meaningful improvements in lung function. This is of interest because poor lung function (low FEV1) is associated with difficult-to-treat disease and worse outcomes.14
Two definitions of clinically meaningful improvement in lung function were applied, with thresholds of ≥100 mL or ≥200 mL increase in pre-bronchodilator FEV1 from baseline to Week 12 constituting a clinically meaningful improvement. Using the 100 mL threshold, 62% of patients in dupilumab groups (combined dose groups) and 49% of patients in combined matched placebo groups achieved clinically meaningful improvement. In total, 49% and 37% of patients, respectively, achieved clinically meaningful improvement using the more stringent definition of ≥200 mL increase in prebronchodilator FEV1. Annualised rates of severe exacerbations were lower among patients with clinically meaningful improvements in lung function (either definition) than among their counterparts without clinically meaningful improvements, both in dupilumab treatment groups and in placebo groups (Figure 2). Significant treatment effects of dupilumab on reducing annualised severe exacerbation rates were apparent in all patient subgroups, with and without clinically meaningful improvements in lung function based on either definition (Figure 2). There was a trend for a greater magnitude of reduction in severe exacerbations, with dupilumab versus placebo, in the patient subgroups with clinically meaningful improvements in lung function. There was a 37–54% reduction among patients with improved lung function (either definition) compared with 35–38% reduction in patients without clinically meaningful improvements in lung function (Figure 2), but the interactions between treatment effect and subgroups were not statistically significant.
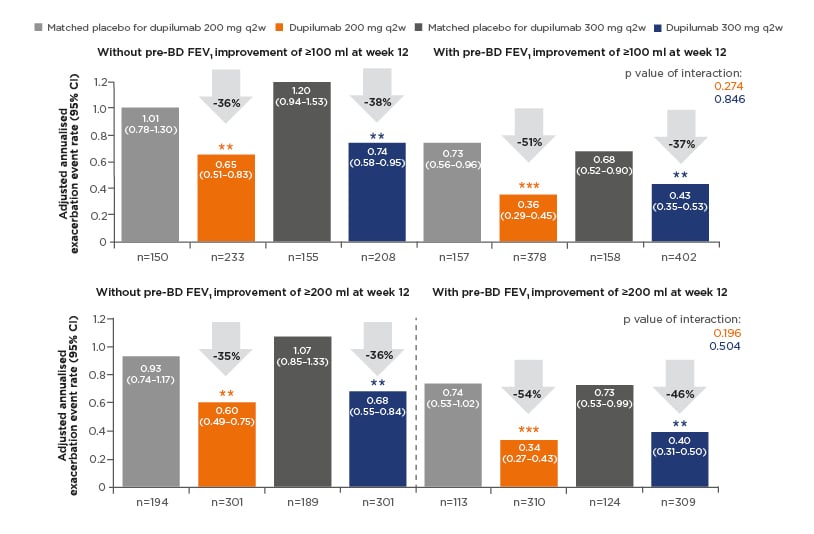
Figure 2: Annualised rate of severe exacerbations during the 52-week treatment period in QUEST in patients with/without clinically meaningful improvements of ≥100 mL and ≥200 mL increases in prebronchodilator forced expiratory volume in 1 second at Week 12 (overall intent-to-treat population).
**p<0.01 versus matched placebo.
***p<0.001 versus matched placebo.
CI: confidence interval; pre-BD FEV1: pre-bronchodilator forced expiratory volume in 1 second; q2w: every 2 weeks.
In the primary efficacy analyses in QUEST, treatment effects on severe exacerbation rates were greater in patients with elevated type 2 biomarkers than in the overall intent-to-treat population.5 This post hoc analysis was repeated in the subgroup of patients with blood eosinophils ≥150 cells/µL and/or FeNO ≥25 parts per billion (which represented most of the overall analysis population: 1,469 of 1,841 patients included in the analysis). Results were similar to the analysis in the overall study population, with significant effects of dupilumab on annualised rates of severe exacerbations seen both in patients with and without clinically meaningful improvements in lung function.
In conclusion, this post hoc analysis showed that higher proportions of patients achieved clinically meaningful improvements in lung function when treated with dupilumab compared with placebo, but the effect of dupilumab on reducing annualised severe exacerbation rate was not dependent on achieving improvement in lung function.
Effect of Dupilumab on Oral Corticosteroid Use in Severe Asthma Patients With Improving Lung Function
Doctor Jorge F. Maspero
Dr Maspero presented a post hoc analysis of the VENTURE trial, exploring the impact of dupilumab on the need for OCS in patients with differing degrees of response in terms of improvement in lung function. Inclusion criteria for VENTURE included regular use of systemic (oral) glucocorticoids (5–35 mg/day prednisone, prednisolone, or equivalent) in the 6 months prior to enrolment, and the study population was therefore a glucocorticoid-dependent population with severe asthma (n=210).7 Patients aged ≥12 years were randomised to receive dupilumab 300 mg q2w or placebo, as add-on treatment to their existing treatment regimen (including bronchodilators, inhaled corticosteroids, and OCS), for 24 weeks. During the study period, patients’ OCS doses were reviewed every 4 weeks and reduced in accordance with a predetermined schedule, dependent on the patient’s optimised baseline OCS dose, until the patient met prespecified criteria indicating that further dose reduction was not acceptable. The primary study endpoint was percentage reduction in OCS dose from baseline to Week 24; key secondary endpoints were proportion of patients with at least 50% reduction in OCS dose at Week 24, and proportion of patients with reduction in OCS dose to <5 mg/day.7
In the overall study population, OCS doses decreased by 70% (least-squares mean percentage change) from baseline to Week 24 in the dupilumab group, compared with a 42% reduction in the placebo group (p<0.001).
In addition, significantly higher proportions of patients in the dupilumab group compared with the placebo group met the secondary endpoints of ≥50% reduction in OCS dose (80% versus 50%, respectively) and reduction in OCS dose to <5 mg/day (69% versus 33%, respectively). Despite greater reductions in OCS use in the dupilumab group, the rate of severe exacerbations was significantly lower and there was a greater increase in mean prebronchodilator FEV1 in the dupilumab group compared with the placebo group.7
In the post hoc analysis that Dr Maspero presented, similar to the analysis of QUEST presented by Dr Hanania and described above, patients were stratified according to their degree of lung function improvement, using thresholds of </≥100 mL and </≥200 mL increases in prebronchodilator FEV1 from baseline to Week 24.
Among patients with ≥100 mL or ≥200 mL increase in FEV1, those in the dupilumab group had 68–70% reductions in OCS dose (similar magnitude of reduction regardless of which FEV1 threshold was used), while patients in the placebo group reduced their OCS doses by only 29–30% (p<0.001 for dupilumab versus placebo; Figure 3).
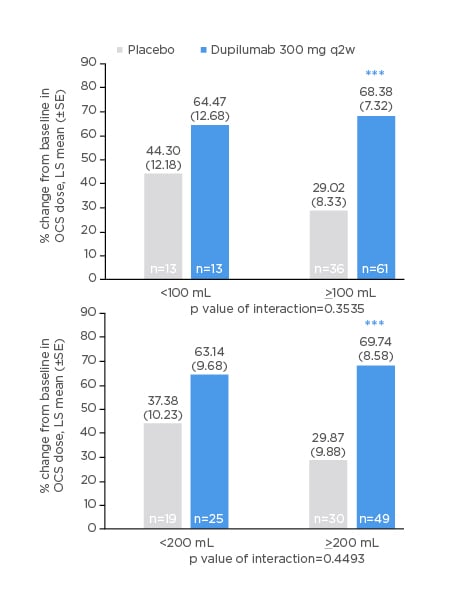
Figure 3: Oral corticosteroid dose reduction (%) from baseline at Week 24 in patients with improvement from baseline in prebronchodilator forced expiratory volume in 1 second of <100, <200, ≥100, or ≥200 mL at Week 24.
***p<0.001 versus matched placebo.
LS: least squares; OCS: oral corticosteroid; q2w: every 2 weeks; SE: standard error.
Among patients with lesser degrees of lung function improvement (<100 mL or <200 mL), reductions in OCS dose in the dupilumab group were similar to those in the improved lung function subgroups (63–64%), but did not reach statistical significance versus placebo.
Similar patterns were observed for other measures of OCS use. In patient subgroups with ≥100 mL or ≥200 mL increase in FEV1, 85–88% of patients in the dupilumab group achieved ≥50% reduction in OCS dose, compared with 49–53% in the placebo group; 79–82% and 40–43% of patients, respectively, reduced their OCS dose to <5 mg/day; and 57–59% and 31–33%, respectively, achieved the maximum OCS dose reduction possible according to their individual dose-reduction schedules (all p<0.05 for dupilumab versus placebo). In the patient subgroups with lower degrees of lung function improvement (<100 mL and <200 mL increase in FEV1), the magnitudes of effect of dupilumab on each of these endpoints were broadly similar, but did not reach statistical significance versus placebo in most cases. This may reflect factors such as different sizes of subgroups (subgroups of patients with <100 mL and <200 mL increase in FEV1 were relatively small compared to the corresponding subgroups with ≥100 mL and ≥200 mL increases in FEV1) because there was no statistically significant interaction between treatment effect and subgroup.
Dr Maspero noted that the findings may be limited by the post hoc nature of the analysis and the low sample sizes in some subgroups. Nonetheless, this analysis indicated that OCS-dependent severe asthma patients, with various levels of improvement in lung function, were able to reduce OCS use during treatment with dupilumab to a greater extent than patients receiving placebo, based on multiple measures of OCS use.
MAT-EU-2000349 October 2020