Meeting Summary
Asthma and chronic obstructive pulmonary disease (COPD) affect millions of people throughout Europe, being one of the leading causes of death in the continent. Both conditions also impose considerable morbidity on patients, adversely affecting individuals’ physical and psychological wellbeing, and their capacity to live and work normally. Asthma and COPD also impose a substantial economic burden on healthcare providers and wider society through both direct and indirect costs of care.
Inhaler-delivered therapy has been central to the successful management of both conditions for several decades. Advances in device technology and understanding of the pathophysiology of both conditions (while theoretically introducing greater flexibility and responsiveness into the repertoire of inhalation therapies) have also added complexity and sometimes confusion into the task of identifying the precise combination of medication and delivery device best suited to the needs of individual patients.
Recently published multinational consensus reports have set out best-practice frameworks for the management of both asthma and COPD. Presentations at the two symposia summarised in this report examined the implications of these guidelines for the treatment of both conditions. Special focus was on dry power inhalers (DPI) as a means of delivering effective treatment that combines ease of use and widespread acceptance among patients, with the potential to reduce medically-related emissions of greenhouses gases compared with pressurised metered-dose inhalers (pMDI).
The authors emphasised the importance of patient partnership in determining the care plan, including the choice of both inhaler device and treatment; the benefits of regular monitoring of adherence to the treatment for both patients with asthma and COPD; and the benefits of simplicity, using one type of inhaler where possible to minimise critical errors in inhalation technique.
Asthma Pharmacotherapy: Are We on Track?
The 2022 update of the Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention proposes a choice of one of the two ‘tracks’ for the management of asthma symptoms and exacerbations (Figure 1).1 There is a misconception that Track 1 is orientated toward treating symptoms at the cost of long-term asthma control, and that the second track may be superior in preventing or controlling symptoms. This is mistaken, since both tracks emphasise the need for maintenance treatment at equivalent steps, and studies that have compared symptom control, measured according to the GINA assessment tool (well-controlled or partly controlled weeks) have not shown a difference between Track 1 and 2 treatments for these endpoints.2
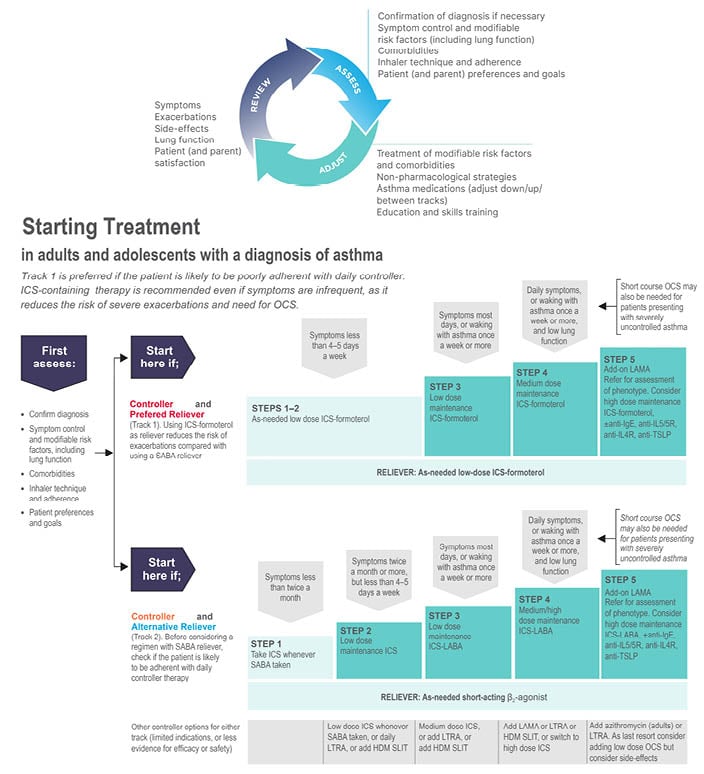
Figure 1: The 2022 Global Initiative for Asthma recommendation advocate a 2-track approach to asthma management.
From Global Initiative for Asthma (GINA)1
Anti-TSLP: anti-thymic stromal lymphopoietin; HDM: house dust mite; ICS: inhaled corticosteroid; LABA: long-acting β-agonist; LAMA: long-acting muscarinic agonist; LTRA: leukotriene receptor antagonists; OCS: oral corticosteroid; SABA: short acting β2-agonist; SLIT: sublingual immunotherapy.
In both tracks, for steps 3 to 5, the recommended maintenance treatment is a dose of inhaled corticosteroid (ICS) plus a long-acting β2-agonist (LABA) according to the severity of disease. The difference, however, is that the reliever used in Track 1 (low-dose ICS plus formoterol instead of a short-acting β2-agonist [SABA]), significantly reduces the risk of severe asthma exacerbations.3-5 It should be noted that the maintenance and reliever therapy (MART) approach with low-dose ICS plus formoterol may not be indicated in all regions. The efficacy of this so-called MART approach, in which a single inhaler is used as both maintenance treatment and for symptom relief, has been consistently confirmed in meta-analysis of clinical trials and in real-world studies.3-5 The reasons that Track 1 is the preferred option for most patients are, firstly, that methods for predicting who might be at risk of an exacerbation are imprecise, and even those whose symptoms are well-controlled are at risk.6 Secondly, as demonstrated in studies of patient adherence with maintenance treatment in asthma, particularly when assessed with electronic inhaler monitoring, only a minority are found to take their maintenance treatment as prescribed.7 Instead, patients are over-reliant on their SABA inhaler, the overuse of which contributes to their risk of a potentially life-threatening attack.8 As further evidence of the similar symptom relief achieved in the two tracks, several studies, including a large 12-month observational study in Europe, had a similar proportion of reliever-free days (61–66%) and a low incidence of high reliever-use days (≤15% of days for one to two as-needed inhalations; ≤2.5% of days for ≥4 as-needed inhalations).9
MART is thus a well-evidenced and highly practical asthma therapy; however, if Track 2 is to be used, patients must be educated and encouraged to use their maintenance inhaler consistently. However, for both tracks, regular appraisal of patients’ inhaler techniques is essential, and forms an integral part of the GINA cycle of care that urges clinicians to ‘assess, adjust, review’, particularly before increasing or decreasing maintenance treatment.
The need for awareness and a willingness to adapt to changed circumstances are not solely the responsibility of patients. High levels of inertia have been recorded among doctors in France treating asthma, with a large proportion of patients remaining on the same ICS dose that they were started on years earlier, and only a small proportion of patients with inadequate asthma control having their controller therapy adjusted.10 There is no reason to think that doctors elsewhere are doing notably better, although this may vary by specialty.11-13 The role of physician inertia as an important potential limitation in asthma treatment needs to be recognised and rectified.
Takeaways from this appraisal of the GINA two-track strategies illustrate that, while each provides a position from which to work, clinical discretion, and good judgement by physicians remains, recognising that:
- Although both GINA tracks aim for (almost) complete symptom control, this is not achieved in a large proportion of patients.
- Predicting which patients are at heightened risk of exacerbations is a complex exercise, as is predicting adherence to controller therapy.
- The choice of a treatment track must factor in the risks associated with use of high-dose ICSs (e.g., osteoporosis, pneumonia, and cataracts), the impact of more frequent (and potentially life-threatening) exacerbations, and the convenience (and resultant possible greater efficacy) of single versus multiple inhalers.
- Patient engagement, and understanding their perspectives and choices, are key determinants of success with both GINA tracks, recognising that the demands on patients differ between tracks. This may influence their buy-in to the proposed approach. Knowing the patient (their attitudes to treatment, preferences for risk, and tolerance of symptoms) is, therefore, an important element in selecting a treatment track.
Treatment of Chronic Obstructive Pulmonary Disease: As Good as GOLD, or Easy As ABCD?
The 2022 report of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) global strategy for the diagnosis, management, and prevention of COPD establishes a systematic framework for the pharmacological treatment of the condition that harnesses both disease phenotype and biomarker information to guide prescribing.
The first step in the treatment pathway is the assignment of patients to Category A, B, C, or D according to their level of symptoms (columns in Figure 2) and risk of exacerbations (rows in Figure 2). This shapes the selection of appropriate drug therapy. For patients in Category A, a short-acting bronchodilator may suffice, but for patients in other categories longer acting bronchodilators are necessary; GOLD prefers a long-acting muscarinic antagonist (LAMA) to a LABA because of the better effect on exacerbations of the former.14
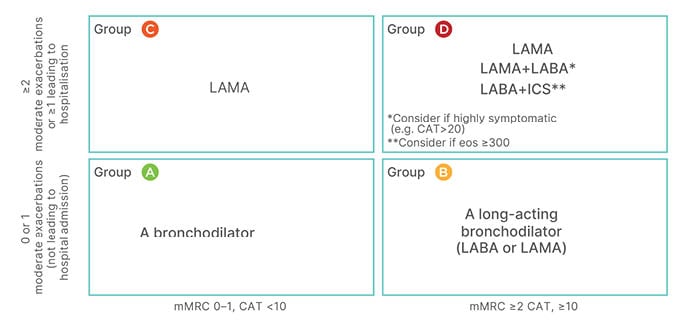
Figure 2: The Global Initiative for Chronic Obstructive Lung Disease 2022 framework for initial pharmacological treatment of chronic obstructive pulmonary diseases stratifies patients according to patients’ level of symptoms (columns) and risk of exacerbations (rows).
CAT: COPD Assessment Test; eos: eosinophils; ICS: inhaled corticosteroid; LABA: long-acting β-agonist; LAMA: long-acting muscarinic antagonist; mMRC: modified Medical Research Council.
Combination of a LABA and a LAMA as an initial treatment is still somewhat controversial, as there is limited evidence from randomised controlled trials (RCT) in newly-diagnosed patients. However, post hoc analysis of treatment-naïve patients in RCTs,15 and the results of the EMAX16 study (which included treatment-naïve patients or those on a single long-acting bronchodilator), support this choice in patients with a marked symptom burden (in essence, Categories B or D). The possible use of an ICS as part of a combination treatment at this stage is predicated on the eosinophil count.
For patients with persistent exacerbations despite initial pharmacological treatment, RCT evidence supports escalation to double and triple therapies involving LAMA, LABA, and ICS. GOLD suggests a pathway for the implementation of this evidence, again using eosinophil count as a guide to the use of ICS (Figure 3).14
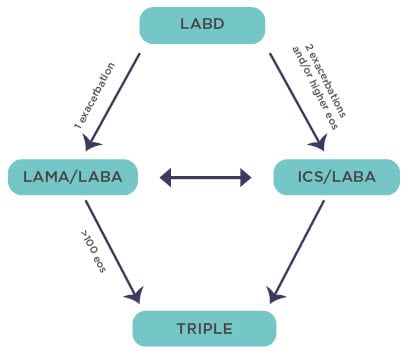
Figure 3: The Global Initiative for Chronic Obstructive Lung Disease recommendations for escalation of inhaled therapy for chronic obstructive pulmonary disease are framed in terms of exacerbation risk and eosinophil levels/counts.
Adapted from Singh et al.14
Eos: eosinophil; ICS: inhaled corticosteroid; LABA: long-acting β-agonist; LABD: long-acting bronchodilators; LAMA: long-acting muscarinic antagonist.
There is now ample RCT evidence indicating that the clinical responses to ICS treatment in COPD are more pronounced in patients with baseline eosinophil counts that are approximately equal to 100/μl,17-20 with a continuous relationship above that threshold such that the exacerbation rate is approximately halved at an eosinophil count of >300/μl.17 The benefits of ICS as an element of triple therapy is provided by the IMPACT trial, which recorded a 42% reduction in on-treatment mortality in patients with COPD at high risk of exacerbation treated with triple therapy versus LAMA plus LABA therapy.21 A reduction in the incidence of exacerbations as seen in the IMPACT trial is likely to have contributed to the survival benefit.22
The pathophysiological mechanism underpinning the relationship between eosinophil status and response to ICSs is thought to involve a greater propensity to T2-mediated inflammation and shifts in the bacterial pulmonary microbiome with, in particular, a reduction in the prevalence of Haemophilus influenzae as eosinophil numbers increase.22
Despite advances in precision-medicine in COPD, the importance of inhaler device selection remains important, as multiple age-related comorbidities can complicate this issue. Worsening hypoxaemia or hypercapnia; cognitive impairment and dementia; neurological or neuromuscular conditions such as Parkinson’s disease, or the aftermath of a stroke; loss of muscle strength in the hands and fingers, or the impact of arthritis and joint pain on finger flexibility and co-ordination may all contribute to increased difficulty of inhaler use and a heightened risk for critical operator errors.23
To address these limitations is a multi-step approach to inhaler selection that starts with an overview of patients’ ability to use any given device in the context of their cognitive function, manual dexterity, and hand strength, and then assesses; medication availability, cost, and reimbursement; and patient preferences for specific devices. Once an initial choice has been made, the healthcare provider needs to demonstrate correct technique and assess patient adoption of it before the chosen device is prescribed for a trial period. Thereafter, regular assessment includes review of adherence, continued use of correct technique, and therapeutic impact, and the imparting of information concerning changes to therapy and/or device selection.23
Other aspects of physical and mental health also need attention. Physical activity levels are a predictor of survival in patients with COPD. While that association does not establish cause and effect, or guarantee that increasing physical activity in patients with COPD will reduce mortality, it does create a strong prima facie case for rehabilitation and exercise programmes designed to foster and sustain greater physical activity.24 The GOLD 2022 report provides a current consensus view of the feasibility and impact of various types of interventions designed to increase physical activity in patients with COPD.25
Exercise may also have a beneficial impact on anxiety and depression, which is noteworthy because both are frequently encountered in COPD, and are associated with poor prognosis, lower forced expiratory volume in the first second after full inspiration, higher (i.e., worse) scores on COPD-specific questionnaires,26,27 and a range of other comorbidities.25 There is no reason to believe that anxiety and depression need to be treated differently in patients who also have COPD, but the finding that COPD patients are 1.9-times more likely to commit suicide than people without is a clear indication that they should not be disregarded.28 Conversely, COPD is widely encountered, but often underdiagnosed and undertreated, in patients with other psychiatric illnesses, and this oversight also needs attention.29
Tailoring Therapy and Empowering the Patient
A common theme running through both symposia was the importance of matching individual patients with asthma and COPD to the type of inhaler best suited to their particular needs. Inappropriate inhaler choice and/or poor inhaler technique impact have on patient health and outcomes in both conditions.30-32 As remarked is an earlier commentary article on this subject: ‘Choosing an inhaler device for drug administration in patients with obstructive airway diseases is as critical as the choice of medication itself, and that in future, the choice of a new compound will be secondary to the need to choose the appropriate inhaler device for the patient.’33
Various detailed comparisons of inhalers underpin this general conclusion. For example, successful use of a pMDI requires slow inhalation and co-ordination with device actuation (unless a spacer device is also used), whereas use of a DPI avoids the need for co-ordination, but requires device preparation and fast inhalation.34 This second requirement has raised concerns that, especially for patients with COPD, the inhalation effort required by a DPI may be infeasible. While optimal peak inspiratory flow (PIF) for good inhalation of medication varies from one DPI model to another, generally within the range of 30–60 L/min, research among patients with COPD from a large Kaiser Permanente database indicates that some 99% of patients with COPD have PIF ≥50 L/min and can, therefore, successfully use an appropriately configured DPI. Careful selection within the DPI class is nevertheless essential.35,36 The demonstration in head-to-head comparison that the Easyhaler® (Orion Pharma, Finland) than Turbuhaler® (AstraZeneca, UK) provides better dose delivery and consistency of dosing at all PIF rates is an example of the sort of data that can guide choice.37
Within the DPI class, the number of preparation steps to use of the device varies from three to 11 according to the model used,38 and the patients’ ability to execute each step in the sequence may affect the success of self-medication. It is thus of some note that both patients with asthma and COPD of varying degrees of disease severity master the five preparatory steps in correct use of Easyhaler® at their first training session.39 Similarly, there can be considerable differences in patients’ reception of different inhalers due to the finger strength required for their operation, with mDPIs requiring the ability to develop markedly more force than most DPIs.40 Patients’ finger strength (and dexterity) should, therefore, be a consideration when selecting an inhaler device. Minimising the scope for error by using only one inhaler device improves clinical outcomes and reduces health care cost compared with multiple-inhaler regimens for patients with asthma or COPD.41,42
Both GINA and GOLD endorse regular review of patient comfort and competence with their inhalers as part of the core asthma/COPD management cycle, with adjustments as necessary. As a result, the voice of the patient is an increasingly powerful factor in discussions about what is the best inhaler device for long-term success.
Lessons relating to patient engagement and empowerment in Finland’s National Asthma Programme and National COPD Programme32,43 include:
- Respiratory disease programmes in general are cost saving, and have the potential to increase respiratory health.
- Self-management and easily accessible information for patients and the public is essential.
- Adherence to management is crucial in chronic disease; promoting adherence may have a greater effect on health than improvements in specific medical therapy.
- Building trust to optimise adherence to treatment requires patient support programmes; repeated patient education to foster high levels of self-efficacy; and healthcare professional behaviours to optimise and promote personalised care.44-47
There is potential for mobile health to facilitate adherence, but evidence to date is mixed.48-50
Inhaled Medications and the Environment: What’s Good for Patients Is Good for the Planet
Growing appreciation of the carbon footprint of respiratory care has led to a heightened emphasis on the potential for greenhouse gas emissions associated with inhaler use. Hereby, the authors reviewed some of the considerations shaping medical practice in response to climate-change concerns.
Prominent among these is the ‘global warming potential’ (GWP) of propellants used in pMDIs. Taking CO2 as a referent and assigning it GWP of one, the chlorofluorocarbon propellants of early pMDIs had GWPs of between four and 10,000. Even 1,1,1,2-tetrafluoroethane, a non-ozone-depleting propellant currently favoured in many pMDIs, has a GWP of 1,300. Other candidate propellants, such as HFA152a, offer further improvement, but nevertheless HFA152a still has a GWP of 138. These data51 identify pMDI propellants, together with industrial use of these gases which are responsible for an estimated 2% of global emissions, as being among the most potent human-generated greenhouse gases.
As a result of these properties of propellants, the carbon footprint per dose (expressed as grams of CO2 equivalent [CO2 e]) of conventional pMDIs is considerably higher than that of propellant-free DPIs. In its 2018 assessment, the Medical and Chemical Technical Options Committee of the Montreal Protocol estimated the carbon footprint of a typical DPI at <20 CO2 e per dose, compared with 200–300 CO2 e per dose for a pMDI using HFC-134a as propellant, and 600–800 CO2 e per dose for a pMDI using HFC-227ea.52 As an example, the Easyhaler® devices generate <5 g/CO2/dose.53
Superficially, there may appear to be a tension between optimising patient care and minimising treatment-related contributions to greenhouse gas emissions. In fact, these objectives are very closely aligned because the best achievable control of asthma minimises disease-related greenhouse gas generation. For example, Kponee-Shovein et al.54 have shown that use of a DPI to deliver SABA medication for the management of mild asthma exacerbations or moderate exacerbations not requiring a doctor’s visit generates close to zero CO2 e, compared with a carbon footprint of 0.3–2.5 kg CO2 e depending on the severity of the exacerbation and the volume of the pMDI. (CO2 e generation for more severe exacerbations is similar for all devices because it is dominated by other sources, such as travel- and hospital-related activities).54
The climate-favourable effect of good asthma control has been further illustrated by Wilkinson et al.55 in work presented as an abstract at the 2021 International Congress of the European Respiratory Society (ERS). A cohort of >200,000 patients aged ≥12 years was identified in the UK Clinical Practice Research Datalink (2007–2017), and stratified according to whether they had well-controlled asthma (defined as use of <3 SABA canisters/year and no exacerbations at baseline) or uncontrolled asthma (≥3 SABA canisters/year or ≥1 exacerbation). Of these, 48% of patients fulfilled the definition of having uncontrolled asthma. Those patients were estimated to generate an average of the equivalent of 191.6 kg/year of CO2 e, a result of the management of their asthma, compared with 63.6 kg/year among patients classified as having controlled asthma. Overall, about two-thirds of emissions were attributable to use of inhaled SABAs although, as in the work of Kponee-Shovein et al.,54 the proportion attributable to inhaled medication declined as the severity of asthma exacerbations increased.
Those findings are amplified by the data from the SABINA study. This overview of SABA use in asthma in European countries identified high levels of SABA overuse (defined as ≥3 canisters/year) in five countries, despite different healthcare frameworks and funding models. Other SABINA data have linked overuse of SABAs with increased risk of exacerbation and mortality in Sweden.8
These findings intimate that reducing heavy dependence on reliever medication and limiting asthma exacerbations and asthma-related hospitalisations benefits both patients and the environment. Proposals for mitigating the environmental impact of inhalation therapy, and therefore stress reduction in SABA use, including reducing need for SABAs in asthma management; replacing pMDIs with DPIs in SABA reliever treatment; and replacing pMDis with DPIs in ICS and ICS/LABA treatment.
Overall Take-Home Messages from the Two Symposia
- Knowing your patient with asthma or COPD contributes to treatment selection; however, patient behaviour ultimately determines the outcome of the treatment selection.
- Effective treatment of COPD utilises precision medicine to help reduce mortality.
- Informed decision making must evaluate the risk of high-dose ICS treatment, more frequent exacerbations, and convenience of one versus multiple inhalers.
- There is a very large difference in carbon footprint between DPI and pMDI inhalers. Poor asthma control and overuse of salbutamol HFC-134a pMDIs are associated with a higher carbon footprint.








