Meeting Summary
At the European Respiratory Society (ERS) Congress 2024, two experts in bronchiectasis, Pieter Goeminne, Department of Respiratory Diseases, Vitaz Saint-Nicholas Hospitals, Belgium, and Michal Shteinberg, Pulmonology Institute and CF Center – Carmel Medical Center; Israel Institute of Technology; and The B. Rappaport Faculty of Medicine, Haifa, Israel, discussed bronchiectasis’ pathogenesis and exacerbations, along with unmet needs regarding diagnosis and treatment. Bronchiectasis is a chronic and progressive inflammatory disease with a rising prevalence. Commonly associated conditions/related comorbidities of bronchiectasis include post-infective diseases and other airway conditions (such as chronic obstructive pulmonary disease [COPD] and asthma), although the cause of bronchiectasis may remain unknown in over a third of patients. Development of bronchiectasis involves the intersection of four pathogenic components: chronic infections, airway ciliary dysfunction, chronic inflammation (mostly neutrophilic), and structural lung damage, commonly known as the ‘vicious vortex.’ In particular, bronchiectasis development, progression, and exacerbation also involve upregulated and dysregulated neutrophil function. Exacerbations in bronchiectasis are marked by symptoms of increased cough, sputum changes, decreased lung function, and fatigue, among others. Careful clinical examination and awareness of bronchiectasis symptoms are needed to properly diagnose and treat the initial condition and prevent exacerbations. Triggers for exacerbations can be endogenous, such as neutrophil or eosinophil increases, as well as exogenous, including the presence of infectious agents and pollution. Research regarding treatment for bronchiectasis is limited, but European guidelines recommend airway clearance techniques and antibiotics during exacerbations. To enable more targeted treatment for bronchiectasis from first occurrence, to limit exacerbations, and during an exacerbation, there are unmet needs for better identification of resistant genes, treatments for pathogens and inflammation, and biomarkers of exacerbation triggers.
Introduction
The chronic and progressive inflammatory disease bronchiectasis is characterised by permanent bronchi dilation and by symptoms such as recurrent bronchial infection, sputum production, chronic cough, dyspnoea, and exacerbations.1 Bronchiectasis prevalence estimates range by geographical region, gender, and age, with indications that prevalence is rising.2-4
One of the most common causes of bronchiectasis, as shown in 16,963 patients in the European Multicentre Bronchiectasis Registry Audit and Research Collaboration (EMBARC) registry, is post-infective disease (21.2%).5 Other causes include COPD (8.1%), asthma (6.9%), tuberculosis (4.9%), and immunodeficiency (4.1%). Of note though, 38.1% of patients in the registry had a cause listed as idiopathic. Bacteria are frequently linked with bronchiectasis, with the most common types found in the EMBARC cohort being Pseudomonas aeruginosa (in 25.1% of sputum samples) and Haemophilus influenzae (23.6%), followed by Enterobacteriaceae (15.9%), Staphylococcus aureus (8.6%), Streptococcus pneumoniae (8.5%), and Moraxella catarrhalis (5.4%). Co-infection was common, with 36.1% of patients having samples positive for at least two pathogens. Occurrence of these infectious agents may differ geographically, for example, H. influenzae infection was lower in Southern Europe, where P. aeruginosa predominated, and was higher in Northern and Western Europe.5
In the first two parts of this symposium, two leading experts in bronchiectasis, Goeminne and Shteinberg, discussed the pathogenesis of bronchiectasis as well as the recognition and control of exacerbations. Such exacerbations can greatly impact a patient’s health-related quality of life (HRQoL).
Bronchiectasis Development and Exacerbation
As shown in Figure 1, four pathogenic components intersect to drive bronchiectasis development, persistence, and exacerbations.6 The first two, chronic infections and dysfunction of airway epithelial cells and their cilia, result in hypersecretion of mucus. Inflammation, the third component, accompanies these phenomena, and together they can result in the fourth component, characteristic of bronchiectasis, permanent airway injury, and dilatation. This opens the airways up to increased epithelial cell damage, infection, and inflammation. As each individual component may not only lead to, but also be brought about by, one of the others, and they do not necessarily occur in any particular sequence, the model of bronchiectasis has moved from being seen as a ‘vicious cycle’ to being recognised as a ‘vicious vortex.’1,7
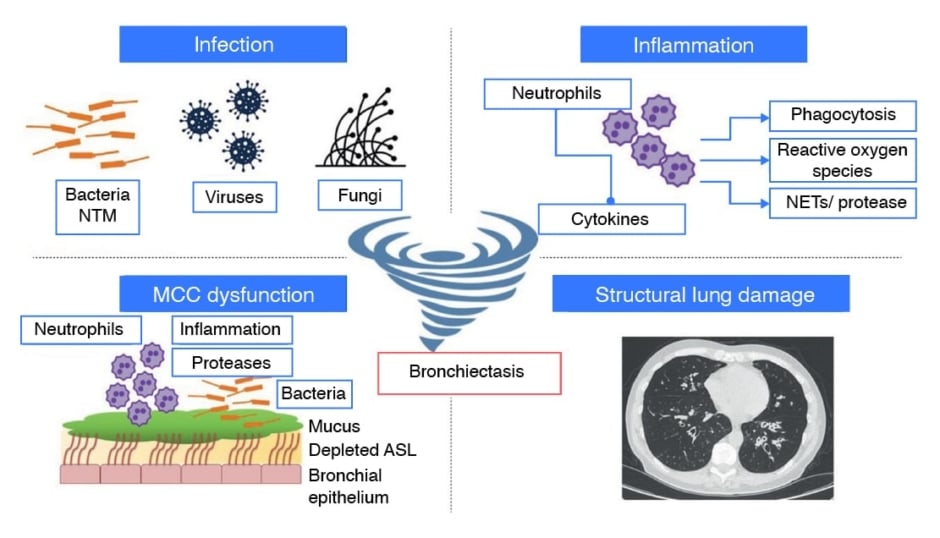
Figure 1: The four pathogenic components of bronchiectasis
Adapted from Chalmers et al., 20236
Pulmonary infection and inflammation, MCC dysfunction, and structural lung damage are common features of the pathophysiology of bronchiectasis regardless of cause. The interactions between these four aspects are not best represented as a cycle; this is due to the fact that each of the individual components can independently affect all
of the other aspects, and therefore it is better described as a “vicious vortex”.
ASL: airway surface liquid; MCC: mucociliary clearance; NET: neutrophil extracellular trap;
NTM: non-tuberculous mycobacteria.
This material has not been reviewed prior to release; therefore, the European Respiratory Society may not be responsible for any errors, omissions or inaccuracies, or for any consequences arising there from, in the content. Reproduced with permission of the ERS 2024. Chalmers JD et al. Basic, translational and clinical aspects of bronchiectasis in adults. Eur Respir Rev. 2023;32(168):230015.
The Role of Neutrophils in Bronchiectasis
Neutrophils usually contribute to acute infection control in a number of ways, including phagocytosis of pathogens, release of granule-resident cytotoxic and microbicidal molecules, extrudation of neutrophil extracellular traps, and generation of reactive oxygen and nitric oxide species. Neutrophils can also have a large influence on the activity of other immune cells, including macrophages and T cells.8 Such actions are usually relatively time-limited; however, a key feature of bronchiectasis is persistent overabundant neutrophil infiltration into the airways that helps drive the viscous vortex through inflammation and promotion of bacterial colonisation.7
Typically, neutrophils survive for only short periods of time; however, in bronchiectasis, their lifespan can be extended in response to bacterial products, cytokines, and interferons.7 Additionally there is a decrease in neutrophil apoptosis and impaired phagocytosis, compared with healthy controls.9
The primary protease involved in tissue damage and remodelling in bronchiectasis is neutrophil elastase. During neutrophil maturation, this protease is activated by dipeptidyl peptidase 1 (DPP1) in the bone marrow, prior to packaging into granules.10 Neutrophil elastase overabundance, along with other proteases, such as cathepsin, matrix metalloproteinase, and proteinase 3 may occur over anti-proteases such as alpha-1 antitrypsin, alpha-1 macroglobulin, secretory leukocyte protease inhibitor, and elafin.11,12 This imbalance, Goeminne posited, may be a key driver of bronchiectasis. Indeed, neutrophil elastase is found in high concentrations in patients with a number of neutrophil-associated lung diseases, including bronchiectasis11 and there is a significant positive relationship between the level of this protease and bacterial load in bronchiectasis.11,13 The pathogenesis of lung damage associated with neutrophil elastase release includes activation of pro-inflammatory cytokines and pathways; impaired immune cell function; goblet cell metaplasia and increased airway mucin expression and dehydration; impaired ciliary motility; and epithelial cell apoptosis and impaired proliferation.11,14
Bronchiectasis Exacerbations
Exacerbations in bronchiectasis are important clinical entities to identify, and while precise definitions may vary slightly according to different guidelines (Table 1), common to all are a number of signs and symptoms, including increased cough and sputum purulence, volume, and/or consistency; decreased lung function; exercise intolerance and/or worsening dyspnoea; increased fatigue and/or feelings of malaise; new or increased haemoptysis; and a change required in current treatment. Also present may be fever, pleurisy, chest pain, and asthenia.15-18 However, Shteinberg discussed that typically, lung infiltrates are not shown on X-ray, and fever may not be present, so these should not be relied upon to make a positive diagnosis. Importantly, Goeminne highlighted, is the role of a healthcare professional in interpreting clinical signs and symptoms as a bronchiectasis exacerbation so that appropriate supportive treatments can be initiated.
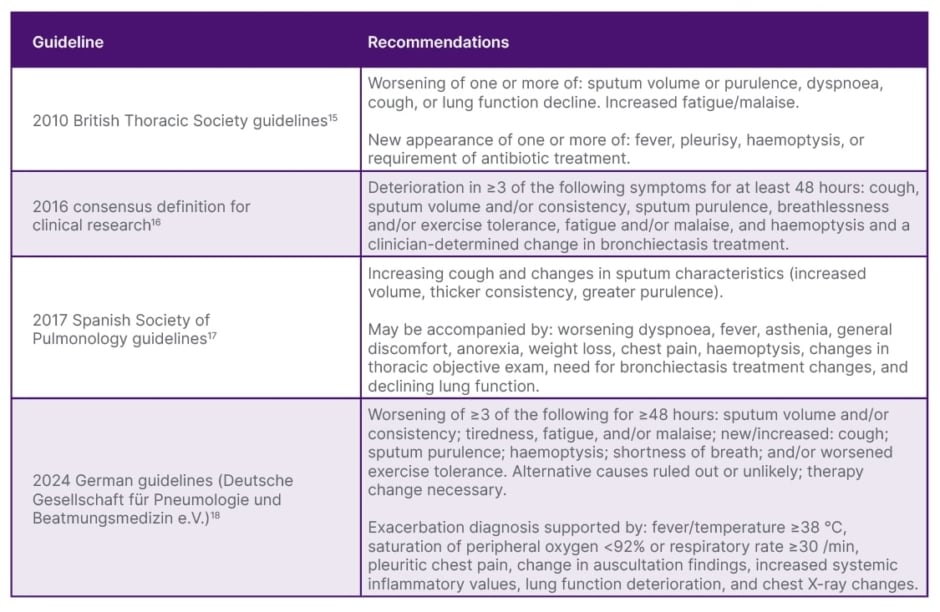
Table 1: Definition of exacerbation according to different guidelines.
In the study utilising EMBARC data, patients had a median of two exacerbations per year, with approximately 40% having at least three per year and over a quarter (26.4%) being hospitalised during an exacerbation in the year prior to the study. Bronchiectasis was generally more severe in Eastern and Central Europe than in other regions. Patients in these parts of Europe also had increased exacerbation frequency, and 57.9% had exacerbations leading to hospitalisation.5 These findings, said Shteinberg, illustrate how common bronchiectasis exacerbations are.
Exacerbation Risk Factors
The highest risk for bronchiectasis exacerbations, as shown in a study including 2,572 patients in Europe, is the number of previous exacerbations. Here, the adjusted incidence rate ratio of a future exacerbation following one exacerbation was 1.81 (95% CI: 1.54, 2.12; p<0.0001), following two exacerbations was 3.07 (95% CI: 2.62, 3.60; p<0.0001), and following three or more exacerbations was 5.18 (95% CI: 4.51, 5.95; p<0.0001), all compared to no exacerbations. Risk of future exacerbations was also associated with type of infection (greater with H. influenzae or P. aeruginosa) and comorbid medical condition (most notably COPD).19
As expected, there is an intimate relationship between lung function and bronchiectasis, with one study showing a decline in the annual rate of forced expiratory volume during the first second (FEV1) of -31.6 mL/year.20 Lower lung function increases the risk of future exacerbations.19 Additionally, more rapid lung function decline is significantly associated with more hospitalisations and/or intravenous antibiotic treatment needed due to an exacerbation.20
Notable is an association between bronchiectasis exacerbations and cardiovascular events; for example, in 1,230 patients with an exacerbation history, both risk of and time to acute myocardial infarction and congestive heart failure was higher compared to 9,484 patients with no exacerbation history.21 There is also a relationship between the number of exacerbations per year and all-cause mortality, which is particularly increased in patients who have ≥3 exacerbations per year.19
Bronchiectasis exacerbations can occur due to exogenous triggers such as pathogens6 and pollution levels.22 These may change regional growth characteristics of the disease through mechanisms such as inflammation, immune dysregulation, and cell damage at the DNA and mitochondrial level.23 There may also be changes in the endogenous microbiome during exacerbations whereby they shift from a less interactive to an ‘antagonistic’ relationship.24 This may occur due to changes in regional growth characteristics of the disease, as well as to changes in microbial immigration and elimination, epithelial cell interactions, and inflammatory cell concentration and activation.25,26
Neutrophils and Eosinophils in Bronchiectasis Exacerbations
Neutrophil activity is a key endogenous trigger of exacerbations. Neutrophil elastase activity is associated with increased exacerbation risk as well as with disease severity, as evidenced by clinical and radiological extent of bronchiectasis, sputum volume, and lung function decline (both occurrence and rate).11,27,28 There is also a correlation between resolution of neutrophil elastase activity after antibiotic treatment for an exacerbation,13 with neutrophil bacterial phagocytosis and killing ability only increasing following antibiotic administration.9
Notably, an association is shown between neutrophil elastase activity and post-exacerbation hospital admission, shorter time to next severe exacerbation requiring hospitalisations or intravenous antibiotic use, and all-cause mortality.11 There is also evidence of a relationship between high neutrophil extracellular trap concentration (>20 mg/mL) and both occurrence of, and shorter time to, bronchiectasis exacerbations.29
While approximately 80% of patients with bronchiectasis are classified as ‘neutrophilic’, with blood eosinophil counts <150 cells/mL, the remaining 20% are considered ‘eosinophilic’, with blood eosinophil counts >300 cells/mL and/or sputum eosinophils >3. Characteristics of such patients include having severe disease, frequent exacerbations, no history of asthma or allergic bronchopulmonary aspergillosis, and possible involvement of P. aeruginosa.30 A relationship is shown between higher eosinophil count and higher annual hospitalisation and exacerbation rates, with the highest levels when blood eosinophil counts are >300 cells/mL. A role of these inflammatory mediators is also shown as treatment with inhaled corticosteroids of patients with eosinophils >300 cells/mL leads to lower annual bronchiectasis-related hospitalisation and exacerbation rates compared to those with low (<100 cells/mL) or interim (101–300 cells/mL) blood eosinophil counts.31
Current thinking points to the existence of a number of endotypes of bronchiectasis exacerbations based on neutrophil and eosinophil expression and bacterial species. For example, one study32 showed a neutrophilic and Pseudomonas dominated endotype, an eosinophilic endotype with no specific dominance, a Haemophilus dominated endotype that had a bacterial response, and a Streptococcus dominated endotype with excess mucus production. Differences between these endotypes were shown in neutrophil elastase activity and in the expression of the pro-inflammatory cytokine IL-1b and the hormone resistin.32 These are exciting findings regarding bronchiectasis, explained Goeminne, “as we are now realising how important inflammation really is.”
Exacerbations and Health-Related Quality of Life
The European study discussed above (n=2,572) investigated the impact of exacerbations on HRQoL utilising the St. George’s Respiratory Questionnaire (SGRQ), which assesses disease-specific health status for asthma and COPD. This is scored on a scale from 0−100 (where a higher score indicates worse HRQoL), with a minimal clinically important difference of around 4.0 points.33 Here, the median SGRQ total score increased according to the number of exacerbations per year, with each additional exacerbation leading to a significant 3.7-point score increase (95% CI: 2.58, 4.87; p<0.0001). Investigation of the data according to hospitalisations found that while there was a 1.4-point increase for each individual outpatient exacerbation (95% CI: 0.57, 2.07; p=0.001), there was a 9.8-point increase for patients with a history of hospitalised exacerbations (95% CI: 6.28, 13.3; p<0.0001).19 These findings echo patient testimony shown as part of the symposium regarding how exacerbations, particularly, can impact their HRQoL.
Management of Bronchiectasis Exacerbations
The American Lung Association suggests a number of ways to help manage bronchiectasis symptoms and limit flare-ups, including quitting smoking and avoiding second-hand smoke; maintaining a healthy diet and staying hydrated; taking medications as prescribed and staying up-to-date with recommended vaccinations; as well as performing daily mucus clearance techniques.34 However, despite prevention and management strategies, around 75% of patients with bronchiectasis experience exacerbations,5 and challenges remain regarding identifying biomarker triggers and resistance genes to help target treatments, and understanding the interactions between triggers and bronchiectasis-associated inflammation (Figure 2).
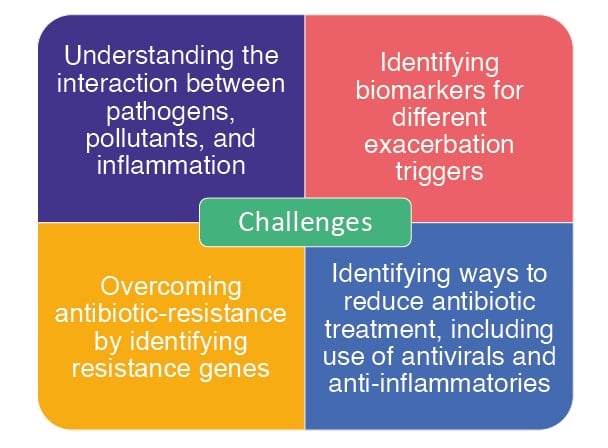
Figure 2: Challenges in the clinical management of exacerbations.24,35
Defined airway clearance techniques, as taught by a respiratory physiotherapist, are part of exacerbation management recommended in British Thoracic Society (BTS) guidelines for all patients with bronchiectasis.36 While these are recommended both during and between exacerbations, Shteinberg discussed how, for patients with mild exacerbations, increased airway clearance should be the first step recommended, reviewing their progress after 2−3 days to ascertain if further treatment is needed. The BTS also recommend using postural drainage, which may be modified and targeted in patients with radiological changes, and, for patients with ongoing airway clearance difficulties, addition of manual techniques, use of positive pressure devices, and enhanced airway humidification/hydration using isotonic or hypertonic saline if secretions are viscous.36
Antibiotics and Other Treatments
According to BTS36 and ERS37 guideline recommendations, the primary treatment for acute bronchiectasis exacerbations is antibiotics. Of note, however, recommendations may be based on low-quality evidence.36,37 Eradication is not a treatment goal, discussed Shteinberg, “rather, the rationale in treating with antibiotics is to reduce bacterial load in the airways, which improves symptoms”.13 According to these guidelines, standard antibiotic treatment is for 14 days, although shorter periods may be considered in patients with variable disease severity or activity.36,37 The BTS guidelines recommend intravenous antibiotics in patients who are ‘particularly unwell, have resistant organisms, or who fail to respond to oral therapy’.36 Antibiotic choice can be guided by the identification of bacterial genus and species,38 and it is recommended that such treatment is always used if a patient is infected with P. aeruginosa.36,37
Shteinberg echoed good practice points from the BTS when discussing her patient consults. These include initiating empiric antibiotics while awaiting microbiology results, taking into account results from previous exacerbations. Once microbiology results are received, antibiotic choice can be adjusted if needed.36
Microbial response to an antibacterial agent is typically assessed using one of several in vitro ‘antimicrobial susceptibility testing’ methods. However, these do not necessarily reflect lung diseases where there may be polymicrobial infection, anaerobic airway niches, and diversity in biofilm growth and phenotype.39 As such, instead of antimicrobial susceptibility testing, Shteinberg discussed how one challenge in bronchiectasis is the need to use genotyping methods to identify antibiotic-resistance genes (Figure 2).24 Another difficulty with regard to antibiotic use is that their primary action in the airways is usually only at the level of the mucus layer, which contains far fewer bacteria than in the underlying biofilm due to inactivation by material released from bacteria therein.40
Studies point to the feasibility of shortening the 14 days recommended for antibiotic use during exacerbations. For example, in one study involving patients with severe pulmonary exacerbations treated with an intravenous antibiotic, 47 were administered 14 days’ treatment and 43 were treated according to bacterial load in sputum samples (bacterial load-guided group: BLGG). Results demonstrated that, on Day 7, 84% of all participants in both groups showed colony-forming units per mL <106. This meant that in the BLGG, bacterial load was considered low enough that treatment could be stopped in 88% of patients on Day 8. In all other participants in the BLGG, treatment could be stopped on Day 11. While clinical recovery was not affected by group, time to next exacerbation was significantly sooner in the 14-day group compared to the BLGG (p=0.0034).41
Studies have also assessed dual antibiotic use, taking advantage of different delivery methods. In one double-blind, randomised study, an oral antibiotic was combined with either an inhaled antibiotic or a placebo in 53 patients with bronchiectasis and chronic P. aeruginosa infection. At Day 21, no differences were found in bacterial load or clinical outcome, which, the authors postulated, may have been related to the finding of more treatment-related wheeze in the inhaled antibiotic group compared with the placebo group.42
Shteinberg also highlighted the challenge of needing to find ways to reduce antibiotic use (antibiotic resistance), for example, by understanding if exacerbations are caused by viral infection, pollutants, or host inflammatory responses (Figure 2).35 Other treatments for bronchiectasis include mucolytics, the use of which is associated with significant improvements in expectoration, sputum volume, auscultatory findings, and FEV1.43 As bronchiectasis is associated with inflammation,1 an anti-inflammatory agent may be prescribed during acute exacerbations. However, in a single-centre, retrospective study (n=43), the use of systemic glucocorticoids in such cases was associated with significantly increased in-hospital mortality (p=0.0474).44
Conclusion
Overall, this symposium highlighted how both patients and their primary healthcare providers need to be educated in identifying bronchiectasis exacerbations and in understanding the impact of exacerbations on a patient’s general health and HRQoL. Daily airway clearance techniques should be taught and escalated if needed during exacerbations, and discussion of antibiotic treatment should include details of how this should result in clinical improvement but may not lead to pathogen eradication. Challenges that remain regarding clinical management of bronchiectasis exacerbations include understanding the interaction between associated triggers35 and identifying biomarkers for such,24 as well as providing clinically evidenced treatment regimens.







