Authors: John Pritchard,¹ Omar Usmani2,3
1. Member of United Nations (UN) Committee on Propellant Medical Usage
2. Respiratory Medicine, Imperial College London, UK
3. Royal Brompton Hospital & St. Mary’s Hospital London, UK
Disclosure: Pritchard acts as a consultant to the pharmaceutical industry in the field of respiratory therapy and has had contracts with many companies, including Altavant, Amiko, Appletree, Aptar, Arcturus, Coelus, Epitome, Nanologica, Spirovant, TianLi, Vectura (pre-2021), Verona, and Zambon. In addition, he is a Director for Cardiff Scintigraphics, iPharma Labs, and Nebu-Flow; received personal fees from Trudell Medical UK, which covered work including a contribution to this publication; and is a minority shareholder in a number of pharmaceutical companies, including AstraZeneca, GlaxoSmithKline, and Pfizer. Usmani reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Chiesi, grants and personal fees from GlaxoSmithKline, personal fees from Napp, personal fees from Mundipharma, personal fees from Sandoz, personal fees from Takeda, grants from Edmond Pharma, personal fees from Cipla, personal fees from Covis, personal fees from Novartis, personal fees from Mereo BioPharma, personal fees from Orion, personal fees from Menarini, personal fees from UCB, personal fees from Trudell Medical, personal fees from Deva, personal fees from Kamada, personal fees from Covis, and personal fees from Kyorin, outside the submitted work.
Acknowledgements: Medical writing assistance was provided by Jem Patel (Trudell Medical UK).
Support: Publication of this article has been sponsored by Trudell Medical International, with content and opinions expressed by the authors based on their clinical and scientific expertise.
Received: 14.03.22
Accepted: 19.04.22
Keywords: Asthma, asthma guidelines, climate change, dry-powder inhalers, inhaler devices, pressurised metered dose inhalers.
Citation: EMJ Respir. 2022;10[Suppl 2]:2-7.
Abstract
Climate change poses a fundamental threat to our livelihood and addressing the crisis through the reduction of carbon pollution is vital. The National Health Service (NHS) in the UK has committed to reducing the carbon footprint from inhaler devices by 50% by 2030, primarily through a reduction in the use of pressurised metered-dose inhalers (pMDI). The development of pMDIs utilising lower global warming potential propellants by 2025 is currently underway. This article focuses on the need to apply an individualised approach for patient outcomes whilst minimising the carbon footprint of their inhaler treatments. The European Respiratory Society’s (ERS) position statement on asthma and the environment introduces the concept of ‘the green’ patient with asthma, where patients’ inhaler usage and technique are optimised to ensure that their respiratory symptoms are well controlled. Measures and education for patients to improve inhaler technique include the prescribing of a suitable inhaler device, ensuring optimal medication delivery through the use of a spacer, whilst minimising adverse drug interactions. The greenest inhaler is the one the patient uses effectively to protect and improve their health without the fear and stigmatism of their carbon footprint.INTRODUCTION
The evidence of climate change has become inescapable.1 From retreating glaciers, shrinking ice sheets, and vanishing snow cover, the most evident signs of global warming trends have occurred since the middle of the 20th century. It is widely acknowledged that global warming correlates with increasing emissions of greenhouse gases.1
In the UK, the NHS Long Term Plan for England 2019 has committed the NHS to reducing greenhouse gas emissions from inhalers, with a target to reduce carbon impacts of inhalers by 50% by 2030, and drive to reduce pressurised metered-dose inhaler (pMDI) prescribing.2-4
The pMDIs use a chemical propellant to produce a therapeutic spray that is inhaled by the patient. In 2014, pMDIs accounted for 0.03% of all greenhouse gas emissions. Demand for pMDIs and other aerosols will grow with increasing population and disease prevalence. Contribution to the greenhouse gas emissions from the Western World could grow to about 15% of the total hydrofluorocarbons by 2035 as other uses are eliminated.5
Among inhalers, pMDIs are the contributor of emissions for two reasons. First, the hydrofluorocarbon propellants they contain contributes to global warming.2,3 Second, pMDIs are the inhalers most frequently prescribed globally; 80% of doses prescribed in the top 10 markets are pMDIs.5
The development of pMDIs containing a low-global warming potential propellant, such as HFA-152a (Koura Global, Boston, USA) and HFO-1234ze (Honeywell, Charlotte, North Carolina, USA),3,6 have the potential to reduce the carbon footprint of pMDIs by as much as 92%.7 Dry powder inhalers (DPI) could cause a significant increase in several other impacts, including human toxicity, marine eutrophication, and fossil depletion.8,9
Moreover, the elements impacting the environmental load of a device are not only linked to the use of propellants, but are also determined by the manufacturing process and waste associated with its use, which necessitates a multifactorial approach when comparing the true ecological footprint of each device.6
The first of these devices is expected to become available in 2025;4 however, a full transition will take time.7
This article will explore the challenges that patients and their clinicians face in balancing treatment outcomes whilst minimising the environmental impact of their inhaler usage.
EUROPEAN RESPIRATORY SOCIETY POSITION STATEMENT
The ERS position statement on asthma and the environment published on World Asthma Day 2021 acknowledges the environmental impact of pMDIs, whilst also outlining the concept of ‘the green’ patient with asthma.8,9 The summary statement highlights the focus of clinical medicine in the past decade on precision medicine initiatives and the need to personalise treatment individualised to the patient. The ERS raises concern about suggestions to switch therapies of patients with stable respiratory conditions from pMDI to DPIs (DPIs are devices that deliver powdered medications without the need for a propellant)9 purely for environmental reasons, as the primary duty of care for any clinician is to put the patient first.5,9
Restricting patient access to pMDIs would be a retrograde step for the respiratory medical community in modern times, especially for those where there are no viable alternatives.8 Incorrect inhaler use in patients with asthma and chronic obstructive pulmonary disease is unacceptably high outside clinical trials, and does not seem to have improved over the past 40 years.10
The optimal choice of the most suitable inhaled therapy is, therefore, a complex decision taken between the healthcare professional and patient.10 Unfortunately, wholesale switching of all pMDIs to DPIs is impractical, economically unfeasible, and may lead to poor patient outcomes.11,12 While many patients are capable of using a DPI, the very young, the very old, and individuals experiencing an exacerbation are three groups who may struggle to generate sufficient inspiratory flows to obtain adequate drug delivery from a DPI.11 The inability of some of these patients to obtain adequate drug delivery with a DPI could lead to poor disease control.8
International guidelines recommend that acute exacerbations should usually be treated with pMDI and spacer; nebulisers are an alternative.9 Most pMDI doses in the top 10 markets are short-acting β agonists (SABA).5 Switching patients from a SABA pMDI to a SABA DPI would increase the cost of this class between 114–566% across the top 10 markets, a cost that would put essential medication out of the reach of many patients.5 In some markets, the cost may also be a barrier to switching other medications from pMDI to DPI. In the UK, a complete switch of corticosteroids to DPI would increase the prescription costs by 21% and by 48% for triple combinations.5
An important reason not to transition is that device switching without a clear medical need can lead to life-threatening asthma exacerbations.6 Patients should not be stigmatised or shamed into changing their inhaler, and the decision on inhaler device should be made with their clinician.12 If a pMDI is the most appropriate treatment for a patient, there are still ways to reduce inhaler carbon footprint, as detailed below.12
THE GREENEST INHALER
This article proposes that the greenest inhaler is one that the patient will use, and will use correctly.8 There is an urgent and pressing need for a broader approach to the problem of the environmental impact of respiratory treatments; notably, involving better overall standards of care and improving healthcare professional and patient training in inhaler technique.10,12
Prescribing a (preventer) inhaler that the patient uses regularly and correctly means that the patient will be controlled at the lowest effective dose, using less propellant and thereby lowering the global warming impact of preventer medication.11
Better disease control will result in: decreased use of rescue medications, which as previously mentioned are primarily SABAs; and fewer asthma attacks and exacerbations, which may result in improved symptoms and quality of life, which in turn reduces morbidity, mortality, and hospital acute care costs.11,12 Poor adherence to medications is never environmentally friendly, so a switch from a pMDI (SABA and preventer) with good adherence to a different device with worse adherence is neither good for the environment nor the patient.8,12
SIX STEPS TO OPTIMISING INHALER USE for THE GREEN PATIENT WITH ASTHMA
Choose the Right Inhaler for the Individual Patient
The assess, choose, and train (ACT) algorithm (Figure 1)8 sets out clear guidelines for inhaler selection based on an assessment of the patient’s ability to inhale slowly and steadily (choose a pMDI, soft mist inhaler, or breath-actuated inhaler), or quickly and deeply (a DPI is a more suitable choice). The use of training devices may prove useful in assessing inspiratory ability.10

Figure 1: Algorithm for choosing an appropriate inhaler device for the treatment of adults with asthma or chronic obstructive pulmonary disease.⁸
*If the patient can perform both inhalation manoeuvres, choose according to patient preference.
†Examples of training devices that can be used to access inspiratory ability are the AIM machine (Vitalograph, Maids Moreton, UK), Clip-Tone (Clement Clarke International, Harlow, UK), Flo-Tone (Clement Clarke International, Harlow, UK), In-Check DIAL (Clement Clarke International, Harlow, UK) inspiratory flow metre, and placebo whistles.
‡Training videos developed by the UK Inhaler Group (UKIG) can be found on the Asthma UK website and RightBreathe.
If the patient is equally proficient at both types of inhalations, choose the type of inhaler that the patient prefers. Once the type of inhaler is selected, consider choosing an option with a lower carbon footprint.9,12 There are usually several options of inhaler to choose from if a patient is best suited to a pMDI. Some inhalers use much more propellant than others; a clinician may wish to select one that uses less propellant.12 Similarly, higher-strength inhalers would require fewer doses per day than lower-strength inhalers. These choices can help to reduce the environmental impact of the patient’s respiratory medication.12
Teach the Patient Correct Inhaler Technique and Reinforce That Training
Use a placebo inhaler to demonstrate technique and train the patient or make use of videos. If not proficient, consider referring the patient to a proficient healthcare professional for training.13 Consider sharing training videos with patients via email or smartphone to help reinforce correct technique.10 At each subsequent visit, ask the patient if they are using their inhaler regularly and evaluate theirtechnique. Correct if necessary.13
Make Every Puff Count
For patients who find it difficult to coordinate breathing and inhaler use, recommend the use of a valved holding chamber, also known as a spacer (Figure 2).13,14 Spacers improve lung deposition, overcome coordination issues, and reduce side effects that result from oropharyngeal deposition of medication.15
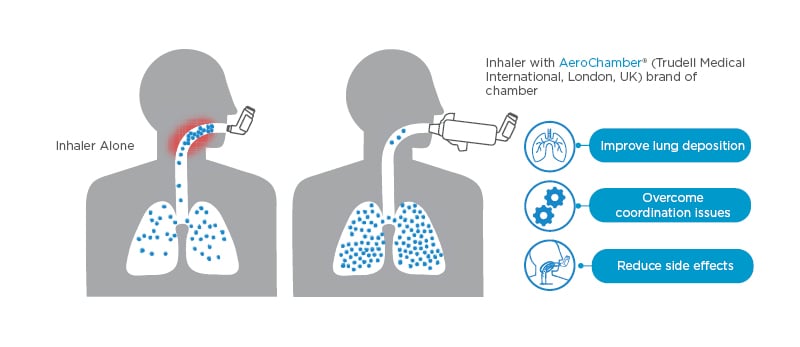
Figure 2: Make every puff count.13,14
Promote Behaviours That Will Help to Improve Disease Control and Reduce Use of Rescue Medications
In patients with asthma, encourage regular use of preventer medication and ensure patients understand their condition and how treatments work. In patients with chronic obstructive pulmonary disease, encourage smoking cessation, exercise, pulmonary rehabilitation, recommend influenza immunisation, and regular use of long-acting bronchodilators.12
Ensure Patients Are Equipped and Able to Self-manage Exacerbations
All patients should have an ‘emergency treatment pack’ with a pMDI, a spacer, and clear and simple pictorial instructions.10,12,16
Ensure Pressurised Metered-Dose Inhalers Are Used Until They Are Empty
Patients should be informed of whether safe disposal or recycling schemes are available for their inhalers, the difference between them, and how to access them. They should also be educated on the importance of ensuring their inhalers are fully empty before submitting them for recycling.12
CONCLUSIONS
Propellants from pMDIs make a small but measurable contribution to global warming, mostly due to the use of rescue pMDIs. The relatively high cost of SABA DPIs and some patients’ inability to inhale with sufficient inspiratory force mean it is not possible to switch all patients to DPIs, and some time will elapse before the widespread availability of next-generation inhalers with a significantly reduced carbon footprint.
For the present, clinicians can help the environment by optimising pMDI use. Measures such as choosing a suitable inhaler, optimising medication delivery of pMDIs through the use of a spacer, teaching and reinforcing correct technique, promoting behaviours that improve disease control, and ensuring patients are equipped for exacerbation self-management, will help to reduce the use of propellants and minimise their carbon footprint.
In summary, the greenest solution for the environmental impact of pMDIs is through optimising inhaler use and ensuring respiratory disease and symptoms in patients are well controlled. The greenest inhaler is the one that a patient can and will use effectively and the greenest patient is the one whose respiratory condition is well controlled.








