INTRODUCTION
The treatment landscape for potentially surgically resectable Stage IIIA non-small cell lung cancer (NSCLC), particularly for adenocarcinoma and squamous carcinoma, has evolved significantly in recent years. Up to now, in patients diagnosed with Stage IIIA NSCLC, the standard approach typically began with neoadjuvant therapies followed by surgical resection, which is considered the cornerstone of treatment. In recent years, in the case of adenocarcinoma and squamous carcinoma, the treatment strategy may vary based on specific molecular characteristics. For instance, epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements may benefit from targeted therapies in adjuvant settings. For potentially surgically resectable Stage IIIA disease, neoadjuvant chemotherapy combined with immunotherapy is being explored to enhance surgical outcomes and improve long-term survival rates.1 Overall, integrating novel immunotherapies and targeted agents into the treatment paradigm is promising, with ongoing clinical trials to optimise outcomes for patients with both adenocarcinoma and squamous carcinoma subtypes. This evolving landscape emphasises the importance of personalised treatment strategies based on tumour biology and patient characteristics, ensuring that the most effective therapies are utilised to improve survival and quality of life.1
MULTIFACETED APPROACH TO POTENTIALLY RESECTABLE STAGE IIIA LUNG CANCER
If Stage IIIA NSCLC is classified as potentially resectable, this categorisation is crucial as it influences subsequent treatment decisions, including neoadjuvant or adjuvant therapies (Figure 1). In histological subtypes, adenocarcinoma and squamous carcinoma can exhibit different biological behaviours and responses to treatment, which are essential for tailoring therapy.2
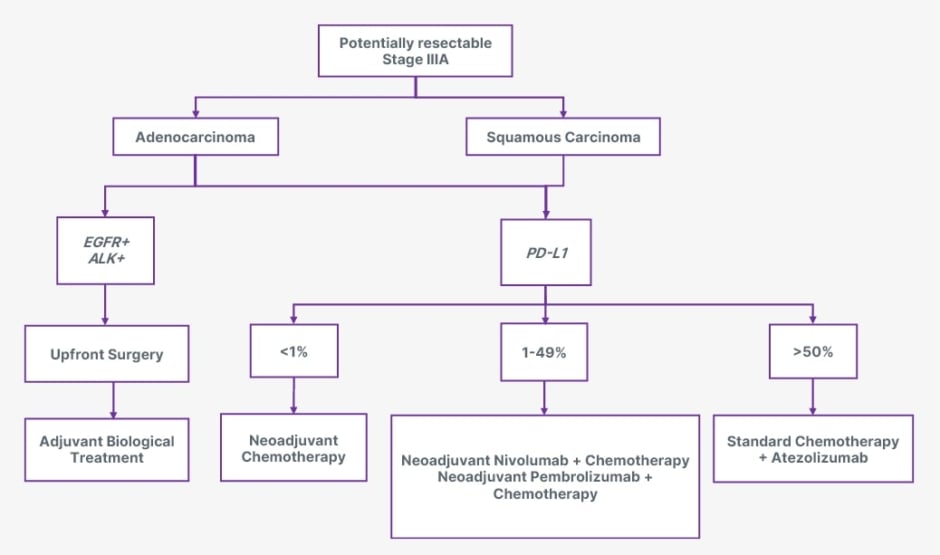
Figure 1: Overview of the treatment landscape for surgically potentially resectable Stage IIIA NSCLC.
ALK: anaplastic lymphoma kinase; EGFR: epidermal growth factor receptor; NSCLC: non-small cell lung cancer; PD-L1: programmed death-ligand 1.
The presence of EGFR and ALK can significantly influence treatment decisions and outcomes. For EGFR-positive tumours, targeted therapies such as EGFR tyrosine kinase inhibitors, like osimertinib, can be highly effective, as they specifically inhibit the activity of the mutated receptor, leading to improved response rates and survival outcomes.3 Similarly, ALK-positive tumours are candidates for ALK inhibitors, such as crizotinib or alectinib, which target specific genetic alteration and have shown significant efficacy.4
Programmed death-ligand 1 (PD-L1) expression levels play a crucial role in treating lung cancer, particularly in determining eligibility for immunotherapy. PD-L1 is a protein that can be expressed on the surface of cancer cells, and its levels are categorised into three distinct groups:
- Less than 1% indicates a lower likelihood of response to PD-1/PD-L1 inhibitors, and patients in this category may not be considered suitable candidates for immunotherapy.
- Between 1% and 49% suggest a potential benefit from immunotherapy, but the response can be variable. Patients in this group may be considered for treatment based on additional clinical factors and the specific immunotherapy used.
- Greater than 50% of PD-L1 expression is generally associated with a more favourable response to immune checkpoint inhibitors.5,6
In potentially surgically resectable Stage IIIA, treatment sequencing is critical. Upfront surgery followed by adjuvant biological treatment involves surgery to remove the tumour as the first step. The goal of upfront surgery is to achieve complete resection of the cancer. Following surgery, patients may receive adjuvant biological treatments, such as immunotherapy or targeted therapy, to address residual disease. This strategy aims to reduce the risk of recurrence by targeting microscopic diseases that could lead to relapse.7 For instance, high PD-L1 expression or specific genetic mutations may benefit from adjuvant therapies that enhance the immune response or inhibit tumour growth. In cases where the tumour is not immediately resectable due to size, location, or involvement of surrounding structures, neoadjuvant chemotherapy aims to shrink the tumour, making it more amenable to surgical resection. Using neoadjuvant chemotherapy can also help assess the tumour’s response to treatment, which can inform postoperative management decisions.6 After surgery, patients may still receive adjuvant therapies to reduce the risk of recurrence further. Both approaches are designed to optimise outcomes by ensuring that the most effective treatments are administered at the appropriate times, based on the individual disease characteristics and overall health status. The choice between these strategies depends on various factors, including tumour resectability, preferences, and the presence of specific biomarkers.7
For patients undergoing neoadjuvant chemo-immunotherapy, the standard regimen often includes a combination of chemotherapy and atezolizumab, an immune checkpoint inhibitor that explicitly targets PD-L1. This approach typically consists of administering four cycles of chemotherapy, followed by atezolizumab for up to 12 months. The chemotherapy component aims to reduce the size of the tumour, increasing the likelihood of achieving a complete resection crucial for long-term survival.1 Immunotherapy enhances the immune response against cancer cells. Targeting PD-L1 helps reinvigorate T cells that the tumour microenvironment may inhibit. This immune activation can lead to a more robust response against residual cancer cells that may remain after surgery.5
The combination of neoadjuvant chemo-immunotherapy aims to facilitate surgical resection and address potential micrometastatic disease that may not be detectable at the time of surgery. In addition to atezolizumab, the treatment landscape includes alternative induction therapies. Nivolumab can be administered with chemotherapy for three cycles and targets PD-1, another checkpoint protein that inhibits T cell activation.8 Similar to nivolumab, pembrolizumab can be combined with chemotherapy for four cycles. Pembrolizumab also targets PD-1 and has shown significant efficacy in improving outcomes in high PD-L1 expression. Notably, pembrolizumab is versatile as it can be utilised not only in the neoadjuvant setting but also in the adjuvant setting after surgery. This flexibility allows for tailored treatment planning based on the individual response to therapy and specific tumour characteristics, such as PD-L1 expression levels.6
FUTURE DIRECTIONS AND RESEARCH GAPS
Despite significant advances in potentially resectable Stage IIIA NSCLC treatment, several critical areas warrant further investigation to enhance patient outcomes and treatment efficacy. Current biomarkers, such as PD-L1 expression and genetic mutations, provide valuable insights but must be fully comprehensive in predicting treatment response and outcomes. Future research should focus on identifying and validating additional biomarkers better to predict patient responses to neoadjuvant and adjuvant therapies. Integrating multi-omics approaches, including genomic, transcriptomic, and proteomic data, could lead to more accurate and individualised treatment strategies. While targeted therapies and immunotherapies have revolutionised the treatment landscape, resistance to these therapies remains a significant challenge. Investigating new targeted agents that address emerging resistance mechanisms is crucial. Additionally, exploring combination therapies that synergistically enhance the efficacy of existing treatments could lead to improved outcomes. Clinical trials should evaluate novel drug combinations and assess their potential to overcome resistance and extend survival benefits. Sequencing neoadjuvant chemotherapy, immunotherapy, and surgical resection requires further optimisation. Research should focus on determining the most effective sequence and duration of therapies to maximise tumour response and minimise adverse effects. Understanding the interaction between different treatment modalities and their impact on long-term outcomes will be essential for refining treatment protocols. Personalised medicine, guided by comprehensive genomic and molecular profiling, holds promise for tailoring treatments to individual patients. Future studies should investigate how personalised treatment plans can be integrated into clinical practice based on a patient’s unique tumour biology and genetic profile. Developing tools to predict patient-specific responses and outcomes will enhance the precision of treatment strategies. Despite advances in treatment, recurrence remains a significant concern in Stage IIIA NSCLC. Research into the mechanisms driving recurrence and metastasis is needed to develop strategies for preventing and managing relapse. This includes studying the role of residual disease and micrometastatic cells that may evade current therapies.9
DISCUSSION
A comprehensive approach for oncologists and surgeons treating potentially surgically resectable Stage IIIA lung cancer should encompass a multifaceted methodology that emphasises personalised treatment strategies based on each patient’s tumour’s unique genetic and molecular profiles.
A growing body of clinical trial data has reshaped the treatment landscape for Stage IIIA NSCLC, particularly concerning integrating immunotherapies and targeted treatments into neoadjuvant and adjuvant settings. For instance, the CheckMate-816 trial provided compelling evidence that neoadjuvant chemo-immunotherapy with nivolumab significantly improved pathological complete response rates compared to chemotherapy alone.8 This trial demonstrated that adding nivolumab resulted in higher rates of tumour downstaging, facilitating surgical resection and improving outcomes for patients with resectable Stage IIIA disease. These findings have begun to influence treatment guidelines, supporting the use of neoadjuvant immunotherapy combined with chemotherapy in clinical practice. In the context of adjuvant therapies, the ADAURA trial revolutionised the treatment approach for patients with resected EGFR-mutated NSCLC. This trial demonstrated that osimertinib, an EGFR tyrosine kinase inhibitor, significantly improved disease-free survival in patients with Stage IB-IIIA NSCLC undergoing complete surgical resection. Based on these results, adjuvant osimertinib has become a standard treatment for patients with EGFR-positive tumours, representing a significant advancement in personalised medicine for this subset of patients.3 Additionally, the IMpower010 study provided important insights into the role of atezolizumab in the adjuvant setting for patients with high PD-L1 expression. The trial showed that adjuvant atezolizumab significantly prolonged disease-free survival in patients with resected Stage II–IIIA NSCLC who had completed neoadjuvant chemotherapy, particularly in those with PD-L1 expression ≥50%. This has led to the incorporation of atezolizumab into treatment guidelines for patients with high PD-L1 expression, marking a critical development in managing Stage IIIA NSCLC.5
Understanding Stage IIIA NSCLC is crucial, as it is characterised by a tumour that may have spread to nearby lymph nodes but is still considered resectable. Genetic and molecular profiling plays a significant role in tailoring treatment. Comprehensive genomic profiling should be conducted to identify mutations and PD-L1 expression levels. The results of biomarker testing should be used to customise treatment strategies, ensuring that therapies align with the specific characteristics of the tumour (Table 1).
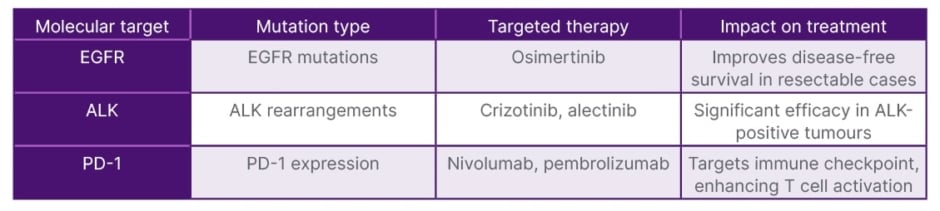
Table 1: Molecular targets and corresponding therapies in potentially surgically resectable Stage IIIA NSCLC.
ALK: anaplastic lymphoma kinase; EGFR: epidermal growth factor receptor; NSCLC: non-small cell lung cancer; PD-1: programmed death-1.
Treatment sequencing is another critical aspect. For patients who are not immediately resectable, neoadjuvant chemotherapy combined with immunotherapy should be considered. After neoadjuvant therapy, the tumour’s resectability should be assessed, and surgical resection should be performed as soon as feasible to maximise the chances of complete tumour removal. Post-surgery, adjuvant therapies should be considered to target residual disease and reduce the risk of recurrence (Table 2).
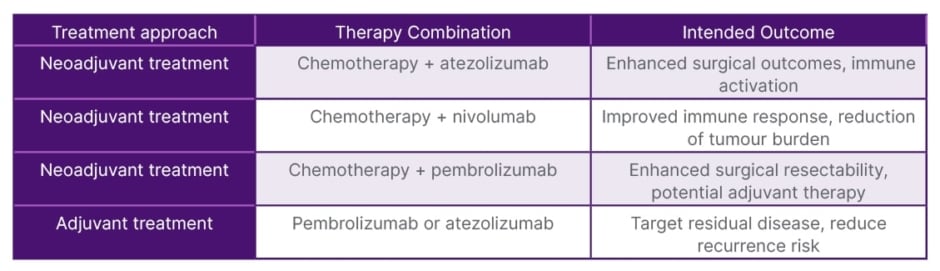
Table 2: Neoadjuvant and adjuvant chemo-immunotherapy approaches for surgically potentially resectable Stage IIIA non-small cell lung cancer (NSCLC).
A multidisciplinary collaboration is essential in managing Stage IIIA lung cancer. Engaging a team that includes medical oncologists, surgical oncologists, radiation oncologists, pathologists, and genetic counsellors is crucial for developing and implementing a comprehensive treatment plan. Discussing various therapies’ potential benefits and risks ensures patients understand their options. A robust follow-up plan should be implemented to monitor for recurrence and manage any treatment-related side effects, which may include imaging studies and clinical evaluations at regular intervals. Supportive care services, including symptom management, nutritional support, and psychosocial counselling, enhance the patient’s quality of life throughout treatment. Continuous education is necessary to keep abreast of the latest research findings and treatment guidelines, ensuring the application of evidence-based practices in patient care.10
A comprehensive approach to managing potentially resectable Stage IIIA lung cancer should encompass not only the clinical and molecular aspects but also the patient’s perspective and quality of life considerations. Understanding Stage IIIA NSCLC requires attention to how treatment impacts patients beyond the clinical outcomes, including their daily lives, psychological wellbeing, and overall quality of life. Incorporating patient-reported outcomes into treatment planning is crucial. Various treatment strategies, including neoadjuvant chemotherapy and targeted therapies, can significantly affect patients’ experiences. For instance, side effects such as fatigue, nausea, and the psychological burden of prolonged treatment can impact patients’ quality of life. Addressing these factors in treatment decisions ensures that therapies are aligned with patients’ preferences and wellbeing.
Furthermore, engaging patients in discussions about their treatment options can help tailor approaches that aim for clinical efficacy and consider patients’ values and goals. A robust follow-up plan should include regular evaluations of patient-reported outcomes to monitor and manage treatment-related side effects effectively. Supportive care services, including symptom management, nutritional support, and psychosocial counselling, enhance patients’ quality of life throughout the treatment process. By integrating these considerations, we can provide a more holistic view of the treatment paradigm, ensuring that therapeutic decisions align with clinical efficacy and patient-centred care.11
CONCLUSIONS
The management of potentially resectable Stage IIIA NSCLC has become increasingly sophisticated, driven by advances in surgical techniques and the integration of targeted therapies and immunotherapies. The standard approach of surgical resection remains pivotal, but the role of adjuvant and neoadjuvant treatments is expanding, particularly for patients with specific molecular characteristics. For instance, targeted therapies and the incorporation of immunotherapies are reshaping the treatment paradigm, offering new hope for improved survival and disease-free outcomes. The importance of personalised medicine, guided by genetic and molecular profiling, cannot be overstated, as it allows for tailored treatment strategies that optimise therapeutic efficacy while minimising unnecessary exposure to less effective treatments. Multidisciplinary collaboration and continued participation in clinical trials will be essential in refining these strategies.







