Abstract
Small-cell lung cancer (SCLC) is extremely sensitive to standard treatments, including conventional cytotoxic chemotherapies and radiotherapy, and has poor prognosis and short survival. Standard therapies have reached a plateau of effectiveness and new therapeutic strategies are needed to improve SCLC patient outcomes going forward. Immunotherapy has revolutionised the treatment of solid malignancies, offering a novel way to harness the host immune system to target malignant cells in patients whose disease may no longer respond to cytotoxic therapy. This review describes the available data for the checkpoint inhibitors, such as anti-cytotoxic T-lymphocyte antigen-4 protein (CTLA-4), anti-programmed cell death-1 protein receptor (PD-1), and ligands (PD-L1 and PD-L2) alone or in combination with first-line chemotherapy or in relapsed SCLC. Several trials investigating immunotherapy in SCLC patients are ongoing and the results are awaited soon. Moreover, further immune checkpoint inhibitors directed against other targets, such as the killer-cell immunoglobulin-like receptor and lymphocyte-activation gene-3, are in clinical development.
Overall, the high expectations from the oncology community are that the drugs under development will offer new and improved treatment options for SCLC patients.
INTRODUCTION
In approximately 15% of cases, new lung cancer patients are diagnosed as having a small-cell lung cancer (SCLC), and in about 70% of these cases the diagnosis is performed at the extensive stage of disease.1 SCLC is closely associated with the intensity and duration of tobacco smoking, and due to the changing smoking patterns of the last decades, the peak of its incidence has been slowly decreasing since the 1980s.1,2 This is due to the change in tobacco habits with the use of efficient filter tips, highly porous cigarette paper, and changes in the composition of the tobacco blend.2
The two stage system, proposed by the Veterans Affairs Administration Lung Study Group (VALG), classified SCLC into limited disease (LD), confined to one hemi-thorax, with or without regional lymph node involvement, which could be encompassed in one radiation field, and extended disease (ED), which is not encompassable in a tolerated radiation field and includes malignant pleural effusion and distant metastasis.3 The VALG staging system is helpful in decision-making but there is a significant difference in prognosis within the LD and ED groups. Thus, the International Association for the Study of Lung Cancer (IASLC) recommended a new staging system based on the American Joint Committee on Cancer (AJCC) TNM for the precise staging of SCLC.4
The multimodality approach is the standard-of-care for LD-SCLC patients with concurrent chemotherapy, platinum plus etoposide regimen, and thoracic radiotherapy. This approach should be offered to all patients with a good performance status and adequate organ function.5 Sequential chemoradiotherapy should be considered for LD-SCLC patients who are not fit for concurrent chemoradiotherapy. Prophylactic cranial irradiation should be considered for LD-SCLC patients who achieve disease control after induction chemoradiotherapy.6 The median overall survival (OS) of LD-SCLC patients is about 15–20 months with 2 and 5-year survival rates of 20–40% and 10–20% respectively.7
The standard first-line treatment for ED-SCLC patients is 4–6 cycles of mainly platinum plus etoposide regimen, followed by active surveillance. Prophylactic cranial irradiation could also be considered for ED-SCLC patients who achieve disease control after induction chemotherapy.6 Median OS for ED-SCLC is about 8–13 months, with 2 and 5-year survival rates of approximately 5% and 1–2%, respectively.7
Overall, despite the fact that SCLC is extremely sensitive to standard therapies, including conventional cytotoxic chemotherapies and radiotherapy, it has poor prognosis and short OS. In fact, SCLC is a very aggressive disease, characterised by a rapid doubling time, high growth fraction, paraneoplastic syndromes, and the early development of widespread metastases, with most of these relapsing within 1 year with an OS <6 months.1 Patients treated with a first-line platinum-based regimen at relapse can be empirically divided into ‘refractory’, i.e., those who progress during first-line treatment; ‘resistant’, i.e., those who show initial response to treatment but progress within 3 months of completing treatment; and ‘sensitive’, i.e., patients who have a relapse-free interval of at least 3 months from completion of treatment.8 The objective response rate (ORR) of second-line therapy ranges from 10–25% for resistant and sensitive disease, respectively, with topotecan being the only globally approved agent in this setting.9
Considering this scenario and to improve outcomes for SCLC patients, new approaches, in particular the potential role of immunotherapy, are under investigation with interesting preliminary results already available. Research in the field of oncology has seen an increase in our ability to harness the host immune system to target malignant cells in a more sophisticated and effective way. This review will focus on the existing data for immunotherapy in SCLC, including immune checkpoint inhibition and exploring correlated emerging biomarkers.
IMMUNOTHERAPY: GENERAL CONSIDERATIONS
Immunotherapy in the management of cancers aims to stimulate immune responses to inhibit the tumour from escaping immune surveillance. To date, two main checkpoints have already been well characterised, including the cytotoxic T-lymphocyte antigen-4 protein (CTLA-4) and programmed cell death-1 protein receptor (PD-1) and ligand (PD-L1 and PD-L2) pathways.
CTLA-4 is a critical negative molecule in the checkpoint pathway that plays an important role in regulating the early activation and proliferation of the T-cell activity peripherally in lymph tissue.10 Two antibodies targeting the CTLA-4 receptor are being investigated in patients with SCLC: ipilimumab and tremelimumab.11
PD-1, also expressed by activated T-cells, engages with PD-L1 and PD-L2 ligands, defining a checkpoint pathway involved in suppressing autoimmunity during T-cell activation, allowing for the immune tolerance of PD-L1 expressed cells at the site of the tumour.10 Among the anti-PD-1 inhibitors, two monoclonal antibodies are in advanced stages of clinical development for SCLC: nivolumab and pembrolizumab. Among anti-PD-L1 inhibitors, another two monoclonal antibodies are in late clinical trials investigation for SCLC: atezolizumab and durvalumab.11
Taking this into account, SCLC is associated with a high non-synchronous mutation burden, a characteristic typically consistent with other cancers exhibiting an excellent anti-tumour response to checkpoint inhibition. This provides a strong rationale for the development of immunotherapy studies in SCLC.12
Although many cancer patients respond well to immune checkpoint blockade and show an improved OS, there are still patients who do not benefit from immunotherapy. Hence, patient selection is a goal to pursue steadily to optimise immunotherapy outcomes.
PD-L1 expression as a biomarker to select patients who could greatly benefit from immunotherapy has been investigated in several solid tumour types, especially in non-small cell lung cancer, even if some patients who are PD-L1-negative based on immunohistochemistry responded to treatment.13 However, while pembrolizumab was approved for the treatment of advanced non-small cell lung cancer patients based on PD-L1 expression (>50% for first-line therapy and >1% for second-line treatment), nivolumab and atezolizumab were licensed for second-line therapy regardless of PD-L1 expression.14 PD-L1 expression is also influenced by a dynamic tumour microenvironment and should be considered as a surrogate of a very complex system. For this reason, the identification of further biomarkers for patient selection, such as the non-synonymous mutation burden, molecular smoking signature, mismatch-repair deficiency of tumours, tumour infiltrating lymphocytes, IFN-γ expression, and intrinsic driver mutations, is necessary to optimise checkpoint inhibition results.15,16
FIRST-LINE TREATMENT
A Phase II randomised trial with three arms investigated carboplatin/paclitaxel regimen plus placebo for 6 cycles; same regimen plus concurrent ipilimumab (10 mg/kg, every 3 weeks), a human IgG1 monoclonal antibody anti-CTLA-4, administered with the first 4 cycles of chemotherapy; or chemotherapy plus ‘phased’ ipilimumab administered with the last 4 cycles of treatment. These regimens were followed by maintenance ipilimumab or placebo every 12 weeks. A total of 130 patients with ED-SCLC were enrolled. The trial’s endpoints included progression-free survival (PFS), immune-related PFS, ORR, immune-related ORR, OS, and safety. The phased schedule of ipilimumab showed better results than the concurrent one and the control arm with grade >3 immuno-related toxicities of 17%, 21%, and 9% for phased ipilimumab, concurrent ipilimumab, and control group, respectively.17
Based on this Phase II randomised trial, phased ipilimumab was investigated in the following Phase III study.18 In this trial, 1,132 patients were assigned to a platinum/etoposide regimen for 4–6 cycles plus phased ipilimumab 10 mg/kg or placebo every 3 weeks followed by a maintenance phase of ipilimumab 10 mg/kg or placebo every 12 weeks until progression. The primary endpoint was OS, and there was no difference between the chemotherapy/ipilimumab versus the chemotherapy/placebo arms. No subgroups demonstrated greater benefit with the addition of ipilimumab versus chemotherapy alone. Grade >3 toxicity was higher in the chemotherapy/ipilimumab arm compared to the control group.18
A Phase II study evaluated phased ipilimumab in combination with a carboplatin/etoposide regimen followed by maintenance ipilimumab every 12 weeks until progression in 42 ED-SCLC patients, of whom 39 were evaluable for safety and 38 for efficacy.19 The primary endpoint was 1-year PFS. In this single-arm study, the evaluation of autoantibody serum levels was planned and correlated with clinical outcomes. Detection of autoantibodies was performed at baseline and during follow-up if clinically indicated and comprised SRY-box 2, anti-human, purkinje cell cytoplasmic antibody type 1, voltage-gated calcium channel antibody, anti-voltage gated potassium channel antibody, anti-nuclear antibody, antineutrophil cytoplasmic antibody, thyroid peroxidase, rheumatoid factors, and anti-muscle antibodies. The 1-year PFS was 15.8%, with five deaths related to ipilimumab. Positivity of an autoimmune profile at baseline was associated with improved outcomes and severe neurological toxicity.
Overall, the lack of benefit from ipilimumab addition to chemotherapy in ED-SCLC might be partially explained by the potential mechanism of chemotherapy-induced immunosuppression, which limits T-cell proliferation, or low T-cell activation within the tumour microenvironment. The treatment benefit correlated to autoantibody analysis; however, as this has been based on a limited number of patients, it should be further investigated.
On the other hand, PD-1 inhibitors targeting tumour-infiltrating lymphocytes, in respect to the anti-tumour activity of CTL-4 inhibitors, act through nonredundant pathways.10
Atezolizumab, a fully humanised, engineered monoclonal antibody of IgG1 isotype anti-PD-L1, was investigated in combination with chemotherapy. The IMpower-133 study was a Phase I/III randomised, double-blind, placebo-controlled trial in which 403 ED-SCLC patients were randomised to receive carboplatin/etoposide plus atezolizumab, at the flat dose of 1,200 mg, or placebo for 4 courses recycled every 3 weeks, followed by maintenance atezolizumab or matched placebo until progression or inacceptable toxicity. Co-primary endpoints were PFS and OS. The median OS was 12.3 months in the atezolizumab group and 10.3 months in the placebo group while the median PFS was 5.2 and 4.3 months, respectively. Atezolizumab did not increase the toxicity related to chemotherapy adding immune-related adverse events which were consistent with the previously reported safety profile of the drug.20 Table 1 summarises the main results of first-line immunotherapy trials.
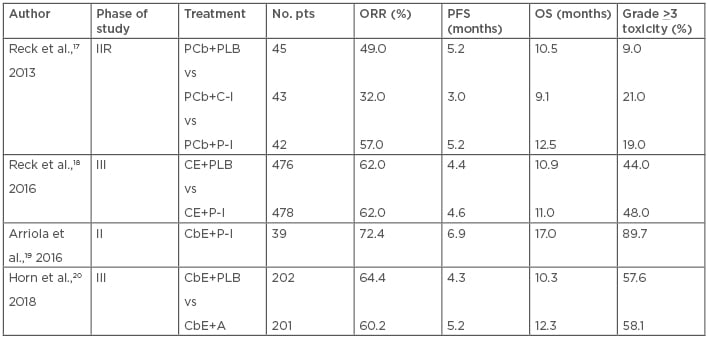
Table 1: Results of the main first-line immunotherapy trials in ED-SCLC.
A: atezolizumab; CbE: carboplatin plus etoposide; CE: cisplatin plus etoposide; C-I: concurrent ipilimumab; ED-SCLC: extensive disease small cell lung cancer; IIR: Phase II randomised; ORR: objective response rate; OS: overall survival; PCb: paclitaxel plus carboplatin; PFS: progression-free survival; P-I: phased ipilimumab; PLB: placebo; Pts: patients.
Considering the results of the IMpower-133 trial, the addition of atezolizumab plus carboplatin/etoposide can be considered as a new potential standard-of-care for first-line therapy of ED-SCLC. However, the results of other ongoing trials with the same study design are awaited and may help in defining whether immunotherapy plus chemotherapy is appropriately transferable to all ED-SCLC patients in daily clinical practice.
STRATEGIC MAINTENANCE APPROACH
To date and in this setting, the results of only one trial are currently available. A Phase II study investigated pembrolizumab, administered at the flat dose of 200 mg every 3 weeks as maintenance therapy. A total of 45 unselected ED-SCLC patients who did not progress to first-line therapy were enrolled. The primary endpoint was PFS, which was 1.4 months with a 1-year PFS of 13.0%. The median OS was 9.6 months with a 1-year OS of 37.0%. The ORR was 8.9%. Tumour tissue, available for 20 patients, was assessed for PD-L1 expression both in the tumour cells and in the surrounding stroma.
A sample was considered adequate for PD-L1 assessment only if there were at least 50 viable tumour cells or 5 viable tumour cells with PD-L1 staining. The stromal interface was considered positive for PD-L1 if a lichenoid pattern of PD-L1 membrane-stained cells surrounding the tumour nests was identified at low power. The median PFS in the eight patients with tumours positive for expression of PD-L1 at the stromal interface was 6.5 months compared with 1.3 months in 12 patients with tumours negative. Serious adverse events included two patients with acute coronary syndrome. The only Grade 3 toxicity that occurred was hyponatraemia in four patients.21
The results reported by pembrolizumab in the small group of patients with tumours positive for expression of PD-L1 at the stromal interface could be used as hypotheses generating in selecting patients who could benefit from this strategic approach. However, the maintenance strategy is still being investigated by other ongoing trials.
SECOND AND LATER-LINE TREATMENT
KEYNOTE-028, a multicohort Phase Ib study, investigated pembrolizumab at the dose of 10 mg/kg, every 2 weeks, for ≥2 years or until progression or intolerable toxicity in 24 patients with PD-L1 expressing (≥1%) ED-SCLC after progression on platinum based chemotherapy, in second or third-line treatment. PD-L1 positivity was defined, with the availability of at least 50 viable neoplastic cells, by membranous PD-L1 expression (>1%) of tumour and associated inflammatory cells or positive staining in stroma. Pembrolizumab showed an ORR of 37.5% with a median PFS and OS of 1.9 and 9.7 months, respectively. Treatment related toxicity was reported in 66.7% of cases with Grade 3–5 adverse events in 8.3% of patients.22
CheckMate-032 is a Phase I/II study evaluating nivolumab as a single agent or in combination with ipilimumab in pre-treated SCLC.23-25 Patients were randomised to receive nivolumab 3 mg/kg every 2 weeks or nivolumab + ipilimumab ([1 mg/kg + 3 mg/kg] or [3 mg/kg + 1mg/kg] every 3 weeks for 4 cycles, then nivolumab 3 mg/kg every 2 weeks). The primary endpoint was the ORR which, in the intention-to-treat population of 216 patients, was higher in the combination arms versus nivolumab alone (23% [nivolumab 1/ipilimumab 3], 19% [nivolumab 3/ipilimumab 1], and 10%, respectively), and this was observed regardless of PD-L1 expression. Tumour PD-L1 expression was categorised as positive when staining of tumour-cell membranes (at any intensity) was observed at prespecified expression levels (≥1% or ≥5% of tumour cells in a section that included ≥100 evaluable tumour cells). Median PFS was 2.6 months in the nivolumab 1/ipilimumab 3 arm and 1.4 months in both the nivolumab alone and nivolumab 3/ipilimumab 1 cohorts. Median OS was 7.7 months (nivolumab 1/ipilimumab 3), 6.0 months (nivolumab 3/ipilimumab 1), and 4.4 months (nivolumab alone). A higher rate of any grade treatment related toxicity was observed in the combination arms (74–80% and 58%; Grade 3–4: 18–30% and 13%, respectively).23 The CheckMate-032 trial also included a Phase II randomised part comparing nivolumab 3 mg/kg versus nivolumab 1 mg/kg plus ipilimumab 3 mg/kg. In the combination and nivolumab arms the ORR was 25% and 11% with a median OS of 7.9 and 4.1 months, respectively. The safety was consistent with previously reported results.24
The results of the subgroup of 109 SCLC patients enrolled in the CheckMate-032 study in third or later-line nivolumab monotherapy 3 mg/kg every 2 weeks, until disease progression or unacceptable toxicity were also reported. The ORR was 11.9% with a median duration of response of 17.9 months. The 6-month PFS was 17.2% with a 12-month and 18-month OS of 28.3% and 20.0%, respectively. Grade >3 toxicities occurred in 11.9% of patients and 3 patients (2.8%) discontinued the therapy due to treatment-related adverse events.25
Based on these results the U.S. Food and Drug Administration (FDA) granted accelerated approval to nivolumab, at the flat dose of 240 mg every 2 weeks, for patients with SCLC progressed after platinum-based chemotherapy and at least one other line of therapy.26
Potential biomarkers of interest in predicting response to checkpoint blockade in SCLC include tumour mutational burden (TMB) and PD-L1 expression. The whole exome sequencing of 211 SCLC patients from the nonrandomised or randomised cohorts of CheckMate-032 was used to evaluate the impact of TMB on efficacy of nivolumab alone or combined with ipilimumab.27 Patients were stratified into low TMB (0–<143 mutations), medium (143–247 mutations), and high (>248 mutations). Within both the nivolumab alone and combined with ipilimumab treatment groups, ORR was higher in patients with high TMB (21.3% and 46.2%) than in those with low (4.8% and 22.2%) or medium (6.8% and 16.0%) TMB. The 1-year PFS was higher in the high TMB group (21.2% and 30.0% for nivolumab monotherapy and nivolumab plus ipilimumab, respectively) compared with the low (not calculable and 6.2%) or medium (3.1% and 8.0%) TMB groups. The 1-year OS was higher in the high TMB group (35.2% and 62.4% for nivolumab monotherapy and nivolumab plus ipilimumab, respectively) than in the low (22.1% and 23.4%) or medium (26.0% and 19.6%) TMB groups. This exploratory data suggested that the high TMB tertile could be considered a predictor of activity, particularly for the combination of nivolumab plus ipilimumab rather than more generally prognostic in patients with SCLC.
Preliminary results from other ongoing trials showed a low activity of immunotherapeutics despite durable clinical activity in certain patients, which needs to be well defined.28-30 Considering all these results together, the role of further line checkpoint inhibitors becomes much less positive, but the results of the ongoing trials should clarify this.
PD-L1 expression was found in SCLC to range from 0–80%.31-34 This high discrepancy between the several case series might be explained by several reasons, such as the different scoring methods, different antibodies, different immunoreactivity in formalin-fixed, paraffin-embedded tissues, delayed or prolonged or inadequate fixation, inadequate fixatives, or pathological interpretive/analytical factors.34
Results from clinical trials investigating immune checkpoint blockade in second and later-line treatment in SCLC patients are summarised in Table 2.
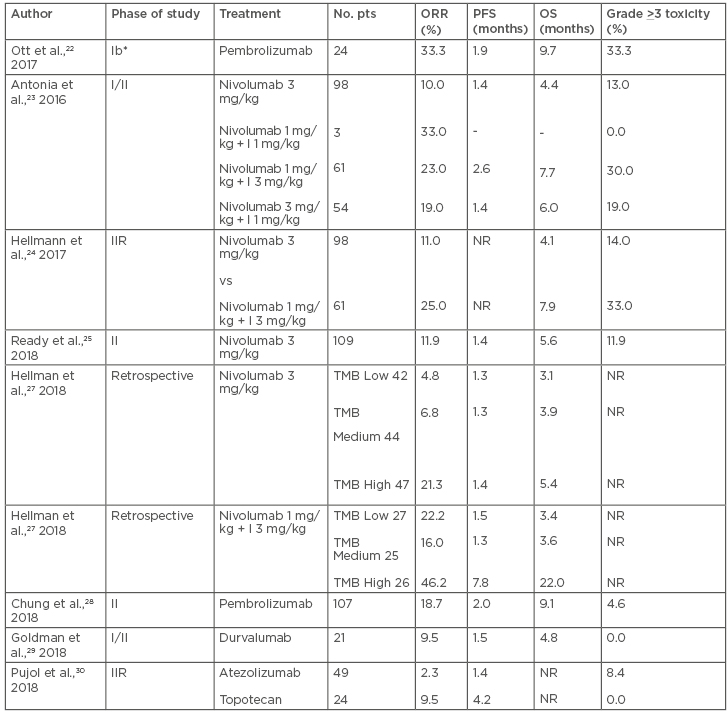
Table 2: Results of the main second or later-line immunotherapy trials in SCLC.
I: ipilimumab; IIR: Phase II randomised; NR: not reported; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; pts: patients; SCLC: small cell lung cancer; TMB: tumor mutational burden.
*Only patients with PD-L1 > 1%.
A press release announced that the CheckMate-331 study did not meet its primary endpoint. The CheckMate-331 was a Phase III randomised open-label study comparing nivolumab versus topotecan or amrubicin in SCLC patients who failed first-line platinum-based chemotherapy. The primary endpoint was OS and was not met, with the secondary endpoints being PFS and ORR.35 The final results will be presented in the forthcoming months.
ONGOING TRAILS
To date, only results from trials addressing first-line ED-SCLC or second and further-line treatment and containing checkpoint inhibitors are available. No data addressing LD-SCLC are available yet. Several ongoing trials are investigating immunotherapy in the first-line setting for ED-SCLC patients. Part E of the Phase I KEYNOTE-011 study is evaluating the safety and efficacy of pembrolizumab in combination with platinum/etoposide.36 The Phase II randomised multicentre open-label REACTION trial is assessing first-line platinum/etoposide with or without pembrolizumab.37 KEYNOTE-604, a Phase III randomised, double-blind study, is enrolling 430 patients to platinum/etoposide plus pembrolizumab or placebo. Co-primary endpoints are PFS and OS.38 CASPIAN is a Phase III, open-label study which completed the accrual of 795 patients who were randomised to either platinum-etoposide versus platinum-etoposide + durvalumab, a human immunoglobulin G1 kappa monoclonal antibody against PD-L1, versus platinum-etoposide + durvalumab and tremelimumab, a fully human monoclonal antibody against CTLA-4. The primary endpoint is OS and the results are still pending.39 CheckMate-451 is a Phase III trial enrolling 810 ED-SCLC patients who did not progress after completion of first-line platinum-etoposide to receive maintenance nivolumab versus nivolumab + ipilimumab versus placebo. Co-primary endpoints are PFS and OS.40 A Phase I/II study is investigating the combination of nivolumab plus ipilimumab with thoracic radiotherapy (30 Gy in 10 fractions) following standard platinum-based chemotherapy. The study will enrol 52 ED-SCLC patients over two parts. Part I of the study will establish the recommended Phase II dose of immunotherapy when combined with thoracic radiotherapy, whereas Part II will estimate the 6-month PFS.41
Several trials are ongoing in LD-SCLC patients. Pembrolizumab and concurrent thoracic radiotherapy with or without chemotherapy are being investigated within a Phase I single-centre study. The aim is to determine the maximum tolerated pembrolizumab dose given in combination with radiotherapy in 80 patients. Secondary endpoints are PFS and safety.42 The randomised, Phase II, open-label STIMULI trial is evaluating the consolidation of nivolumab and ipilimumab following completion of chemo-radiotherapy. Patients will be randomised to an induction phase of nivolumab 1 mg/kg + ipilimumab 3 mg/kg, every 3 weeks for 4 cycles, followed by a maintenance phase (nivolumab 240 mg every 2 weeks for 12 months) or observation. Co-primary endpoints are OS and PFS.43
In second or later-line SCLC management, several immunotherapy trials are ongoing. A multicentre, randomised, open-label Phase II study is enrolling 98 patients to pembrolizumab versus topotecan. Although PD-L1 expression determined at baseline is mandatory, the enrolment will occur regardless of PD-L1 status. Crossover to pembrolizumab is allowed in the topotecan arm at progression. The primary endpoint is PFS.44
Another randomised Phase II trial is currently enrolling patients with progressed SCLC to durvalumab plus tremelimumab with or without radiotherapy. Co-primary endpoints are PFS and ORR.45 Table 3 summarises the main ongoing trials in SCLC patients.
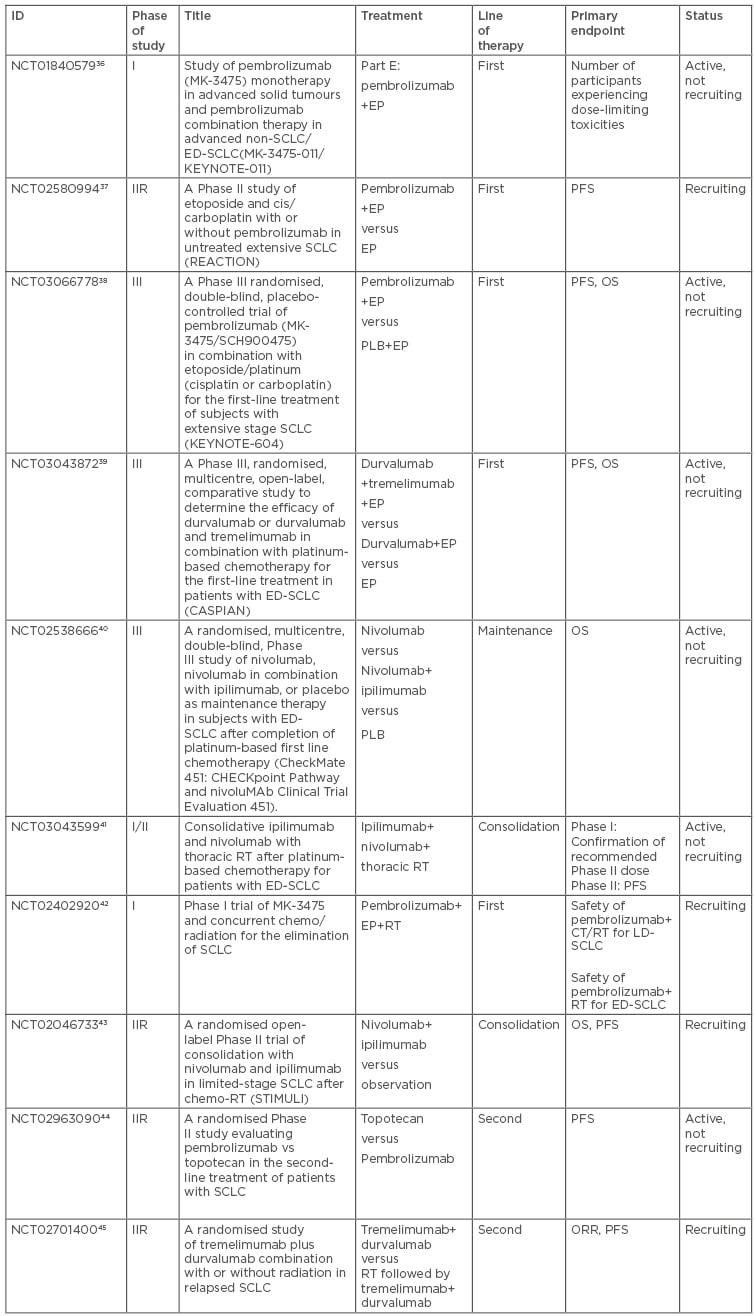
Table 3: Characteristics of the main ongoing trials in SCLC patients.
ED: extensive disease; EP: platinum plus etoposide; IIR: randomised Phase II trial; LD: limited disease; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; PLB: placebo; RT: radiotherapy; SCLC: small cell lung cancer.
CONCLUSION
Immunotherapy through the administration of anti-CTLA-4 and anti-PD-1/PD-L1 agents, alone or in combination with first-line chemotherapy or in relapsed SCLC, may become a new option for the management of SCLC. To date, nivolumab has already been approved by the FDA for the third-line treatment of SCLC.26 Moreover, the combination of atezolizumab plus carboplatin plus etoposide improved the OS of SCLC patients in first-line setting.20 Furthermore, several clinical trials investigating checkpoint inhibitors are still ongoing. Moreover, to optimise the results of immunotherapy the selection of patients based on immune biomarkers is of paramount importance.
In addition, further immune checkpoint inhibitors directed against other targets, such as the killer-cell immunoglobulin-like receptor and lymphocyte-activation gene-3, are in clinical development.16
In the next few years, there will be an increasing amount of new data produced through immunotherapy research, and hopefully the research community’s high expectations will be met. The author further hopes that these results, together with the drugs under development, will offer new and improved treatment options for SCLC patients.








