Abstract
Asthma is the most common chronic condition in Western countries. Affecting 1 in 7 children and 1 in 12 adults, asthma is responsible for >350,000 avoidable deaths every year. While most children who develop symptoms of asthma are <5 years of age, the disease is frequently misdiagnosed or not suspected in infants and toddlers. In addition, the prevalence of asthma is different in males and females throughout their lifespan. While boys are more likely to develop asthma than girls, this pattern is reversed after puberty. This indicates that sex-specific factors, such as fluctuations in hormone levels, play a role in the disease’s pathogenesis. In this review, the authors discuss recent advances in diagnostic tools for asthma in both adults and children, as well as the influences of BMI, environmental exposures, socioeconomic factors, and sex hormones in the disease’s pathogenesis. The review will show that both experimental and epidemiological evidence suggest that circulating sex hormone levels are important contributors to asthma symptoms in post-pubertal females, while their role in males and children has not been yet established. In addition, the mechanisms associated with these hormonal influences on airway inflammation and hyper-reactivity have not been yet elucidated. The authors conclude that different factors affect asthma rates and severity in children and adults, and that more research needs to be conducted to identify the specific contributions of sex hormones. These will allow the development of more personalised asthma treatment strategies for men and women at different stages of life.
INTRODUCTION
Asthma is a chronic inflammatory disorder of the airways, characterised by obstruction of airflow, excessive mucus production, and airway hyper-reactivity. While asthma may commence at any point in life, most symptoms begin in early childhood. The pathogenesis of the disease involves a number of mechanisms in different cell types, most of which remain understudied. Inflammation is a key component of asthma and involves early and late phase responses, which are characterised by the recruitment of specific immune cells.
During puberty, asthma symptoms can ameliorate or worsen, depending on a number of factors.1 The observed sex differences of asthma incidence in children <18 years of age in the USA (9.2% in boys versus 7.2% in girls) and in adults (6.2% in men versus 10.4% in women)2 suggest that hormonal changes occurring during puberty may contribute to the increased incidence in adult women. This is further supported by studies indicating variation in the severity and frequency of asthma symptoms throughout the menstrual cycle.3
PAEDIATRIC ASTHMA: DIAGNOSIS AND MANAGEMENT
A large number of children who develop symptoms of asthma are <5 years of age.2 Despite this, the disease is frequently misdiagnosed or not suspected in infants and toddlers.4 Establishing the diagnosis of paediatric asthma involves a careful process of history taking, physical examination, and diagnostic studies. Coughing and wheezing are some of the most common symptoms in childhood, but shortness of breath, chest tightness, chest pressure, and chest pain are also reported. In addition, poor school performance and fatigue may also present as a result of sleep deprivation from nocturnal symptoms.
Effective asthma management in children requires preventive and proactive approaches.4 Currently, most asthma-related visits are for urgent care. Routine follow-up visits for patients with active asthma are recommended every 1–6 months depending on the severity of asthma and management strategies, which are determined based on age group and symptoms.4 Once the severity of asthma has been assessed, treatment is initiated and efforts are made to keep the asthma in the well-controlled category. Step-wise treatments are effective in most patients: if the symptoms aggravate, therapy is stepped up, and vice versa if symptoms improve.5
Clinicians use a variety of tools to diagnose asthma; for example, measurement of peak expiratory flow rate is a useful indicator of airflow obstruction, the hallmark symptom of asthma. Spirometry, which additionally measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), can also be used to document airflow obstruction. Spirometry can detect airflow obstruction in the presence of normal peak expiratory flow. These tests are useful tools when diagnosing asthma in adults, but the situation is different when it comes to dealing with asthma in children. In children, parents have to look out for symptoms like unresolved chronic cough, wheeze, poor sleep, irritation, and/or being restless. Thus, the first step in diagnosis is compiling a proper patient history. This is only possible if parents monitor symptoms and visit their primary care physician to get their child checked and if attention is being paid to additional factors that can accompany traditional asthma symptoms. In this regard, a number of studies have explored a variety of factors that can influence asthma diagnosis in children. These studies are summarised in Table 1.6-13
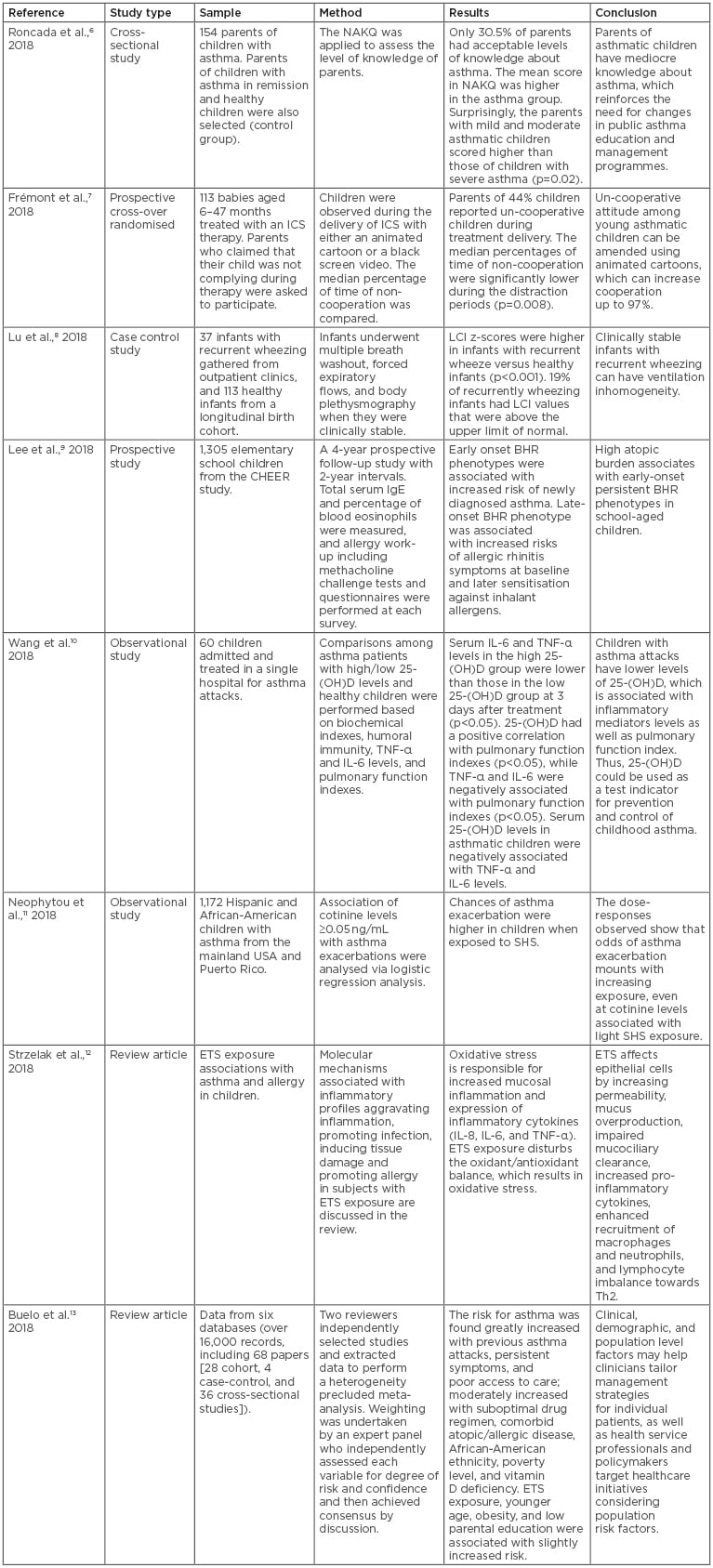
Table 1: Clinical studies on paediatric asthma risk and management.
BHR: bronchial hyper-responsiveness; ETS: environmental tobacco smoke; ICS: inhaled corticosteroid; LCI: lung clearance index; NAKQ: Newcastle Asthma Knowledge Questionnaire; SHS: second hand smoking.
Recently, studies were conducted to determine if parents of asthmatic children had satisfactory levels of knowledge when it comes to their child’s condition.6 The results revealed that most parents had an unsatisfactory level of knowledge about asthma, and suggested that changes should be made in public asthma management programmes to improve parents’ awareness of their child’s condition, therefore allowing them to appropriately manage their child’s care. It was also noted that, even after asthma was diagnosed in children, the disease was not well controlled, partly due to non-adherence. On the contrary, adherence was significantly associated with well-controlled asthma, and adherence to proper medication regimens was found to be necessary for obtaining maximum therapeutic benefits.6 These results further support the notion that parents should be well aware of all aspects of asthma health care. In addition, the naturally non-cooperative attitude of children can affect asthma treatment efficacy. Recently, a study of cooperation in children aged 6–47 months was conducted.7 The study found that poor cooperation among young children with asthma was associated with poor prognosis. However, the study also revealed that cooperation could also be improved using various techniques, such as music, toys to distract children, and animated cartoons, which increased cooperation up to 97%. On the contrary, cooperation and adherence in adults was not found to be a major issue in this report.7
Because asthma in children presents with various nonspecific symptoms, it is difficult to assess if a child has asthma. A recent report showed that ventilation inhomogeneity is present in clinically stable infants with recurrent wheezing, and their spectrum curve indices indicated bronchodilation may be useful for the assessment of bronchial reversibility in children with asthma.8
In one recent study, the early-onset persistent bronchial hyper-responsiveness (BHR) phenotype in school-aged children was found to be associated with high atopic burden and increased risk of newly diagnosed asthma, whereas the late-onset BHR phenotype was related to later sensitisation and allergic rhinitis symptoms.9 Some studies have also given their input on diagnostic criteria for asthma, which can be very helpful in children in whom spirometry cannot be performed accurately, including the use of potential biomarkers.14 In some children with asthma attacks, circulating levels of 25-(OH) vitamin D were decreased, which was associated with the inflammatory mediators IL-6 and TNF-α, as well as altered pulmonary function.10 This study showed that patients with allergic asthma had distinctively reduced vitamin D levels, suggesting that 25-(OH) vitamin D could be considered a biomarker for the prevention and control of childhood asthma. However, the use of vitamin D as a biomarker for asthma remains controversial and has not been established or validated in the field.15
PAEDIATRIC VERSUS ADULT ASTHMA
Many risk factors for the development of paediatric asthma are different to those found in adults. These differences will call for alternative preventive measures in both patient groups. For example, prevention of occupational exposures, smoking, and allergens/pollutants can help in adults, whereas avoiding certain foods and environmental exposures, such as second-hand smoking (SHS), can help in children. In both cases, asthma is mostly triggered by allergens and air pollutants. Thus, whenever a child presents to the clinic with unexplained chronic cough or wheeze, questions regarding these triggers must be asked.
A study analysing dose–response relationships of asthma and SHS in children indicated increasing odds of asthma outcomes related with increasing exposure to cotinine, even at cotinine levels associated with light SHS exposure.11 A recent review of environmental tobacco exposure and the effect of this exposure on asthma and allergy in children indicated that oxidative stress imbalance resulting from tobacco smoke exposure can lead to mucosal inflammation and increased expression of inflammatory cytokines.12 In addition, direct cellular effects of environmental tobacco smoke (ETS) on epithelial cells resulted in increased permeability of mucous membranes, mucus overproduction, impaired mucociliary clearance, increased pro-inflammatory cytokine and chemokine secretion, enhanced recruitment of macrophages and neutrophils, and disturbed lymphocyte balance towards Th2.12 These mechanisms could play a major part in the pathogenesis of asthma and allergy in children; therefore, avoiding passive smoke exposure is a good preventive measure. Other factors contributing to the increased risk for asthma in children are previous asthma attacks, persistent asthma/allergy symptoms, ethnicity, and poor access to care.16 In this regard, a recent review of six databases found a moderately increased risk for asthma as a result of suboptimal drug regimen, comorbid atopic/allergic disease, African–American ethnicity, poverty level, and vitamin D deficiency.13 Moreover, ETS exposure, younger age, obesity, and low parental education were also found associated with slightly increased risk of asthma in children.13
Exposure to both indoor and outdoor air pollutants has also been associated with asthma in both children and adults.17 For example, cooking behaviours, especially in underdeveloped nations, may contribute to the burden of particulate matter exposure in the homes of children with asthma and thus to asthma symptoms.18 A recent study suggested that these act by altering molecular pathways, resulting in both respiratory and cardiovascular disease.19 In clinical studies using ozone exposures, investigators found varied responses in both children and adults, and many studies were also reviewed to see if ozone exposure contribute towards asthma pathogenesis.20 For adult-onset asthma, long-term ozone exposure was found to be associated with varied increased risk in men versus women; however, the mechanisms of action have not been explored in detail.21
Genetic risk factors have also been proposed to play a role in asthma development. Accordingly, genetic contributions to asthma have been studied in various populations that identified specific gene associations and epigenetic markers associated with asthma development when the patients are <18 years of age.22,23 One example is a study evaluating potential candidate gene–environment interactions in allergic symptoms and childhood acute lymphoid leukaemia, which found an inverse relationship between these two phenotypes.24 The authors suggested that this inverse association between acute lymphoid leukaemia and asthma could be limited to children carrying certain genetic polymorphisms.24 However, none of these genes have been shown to play a role in the development of asthma in adults alone. While adult lung function-related genetic variants were associated with childhood lung function, studies revealed that there was also an environmental component.25 In this regard, some of the factors that modified the observed effects were maternal atopy, smoking during pregnancy, cigarette smoke exposure during childhood, and birth weight.25 With regard to allergic and immune factors, a study found that children who had higher IgE levels at birth were naturally more prone to developing asthma.26 Also, patients with monovalent IgE allergies to moulds were also found to have higher risk for asthma than patients with other allergies.26 Their asthma was often found to be more intense and less controlled compared with that of patients with other types of allergies.
Regarding maternal factors, studies evaluating the mother’s immune status during pregnancy,27 maternal exposures,28 perinatal exposures,29 and the microbiome30 suggest that the process of asthma development begins in utero and is independent of allergy. Accordingly, developmental exposure to endocrine disruptors, such as bisphenol A, can also alter immune function and contribute to the development of allergy and asthma, together with other diseases, such as Type 2 diabetes mellitus and cancer.31 Thus, preventing exposure to bisphenol A may prove fruitful in avoiding the development of asthma in children, but its role in the adult population has not been studied in detail.
While many patients experience changes in asthma throughout life, asthma does not end at a certain age bracket, resulting in long term consequences in different age groups. Children with frequent asthma attacks and allergies, especially those who become adult smokers, are the most vulnerable group to show a decline in lung function when compared to normal non-asthmatic patients. Recently, the association between asthma and other chronic obstructive diseases in the adult and paediatric population was studied in 12,594 adults.32 When chronic obstructive pulmonary disease and lung function were studied in a cohort of 53-year-old patients and correlated with childhood lung function, asthma, and smoking, it was evident that active asthma in adults is a dominant mediator in associations between childhood asthma-related risk profiles and middle-age lung function/chronic obstructive pulmonary disease.33 Thus, appropriate interventions at a younger age may prevent patients from developing more severe obstructive respiratory disease in adulthood.
SEX DIFFERENCES IN CHILDHOOD AND ADULT ASTHMA
From the above discussion, it is obvious that differences exist in the incidence, risk factors, preventative measures, pathogenesis, presentation, prognosis, and treatment of asthma among children and adult populations. It is clear that a significant amount of work has been done to uncover the root cause of these differences to tailor preventive and treatment options for children and adults; however, little has been done to explain the differences observed in asthma incidence and presentation between male and female individuals. In the next section, the authors summarise the available literature describing this phenomenon and the potential mechanisms associated with it. The causes for the observed sex differences in both the paediatric and adult groups remain unknown.
Data on asthma prevalence from the U.S. Centers for Disease Control and Prevention (CDC) indicated that in 2016 about 11.2% of the USA ‘young teen’ population (aged 12–14 years) had asthma.2 A recent population study in children and adolescents reported that the prevalence of asthma (‘asthma ever’ and ‘wheezing in the past 12 months’) in middle school children (aged 13–14 years) was significantly higher than that in elementary school children (aged 6–7 years).34 This study also found that the severity of asthma in girls was higher than that in boys aged 13–14 years.34 One factor affecting these statistics is obesity and BMI; however, studies on this topic remain controversial. A study linking BMI and asthma prevalence in children aged 7–14 years showed that higher BMI increases the risk for asthma in females, whereas the inverse was true for males.35 A similar study in adults showed that obese women had significantly higher rates of asthma than obese men and that these rates were affected by smoking status.36 When lung function parameters were compared in children and teens with varied BMI, a lower mean FEV1/FVC was observed among students with asthma and high BMI, which was more pronounced in boys than in girls, indicating that BMI increases the risk for asthma in boys at a younger age.37 Together, these studies highlight the importance of BMI in paediatric and adult populations when assessing asthma, and the need for additional research in these areas to address discrepancies among studies. These data also underscore the potential role of obesity and smoking as modifiable asthma risk factors that most strongly affect adolescent women.
With regard to asthma control and quality of life, a study conducted in a Chinese cohort showed that only a minority of asthmatic adolescents reported well-controlled asthma and that poor asthma control and female sex were risk factors for low health-related quality of life.38 In addition, this study reported different rates for asthma attacks and hospitalisations in elderly, middle aged, and young groups, but in all cases women were more prevalent and significantly more likely to have a positive allergen test than men.38 A study focussed on the unequal prevalence of asthma in men and women showed that both the male predominance prevalence before puberty and the ‘sex-shift’ towards females after puberty onset were stronger in multimorbid patients who had asthma and allergic rhinitis concurrently.39 Other studies of asthma comorbidities found adverse associations between cardiovascular disease vulnerability and the timing of asthma onset in adolescent boys.40
ROLE OF SEX HORMONES IN ASTHMA
Studies in animal models have suggested the involvement of sex hormones in mechanisms of lung inflammation and asthma.41 In particular, recent studies using tamoxifen in equine asthma models have highlighted the contributions of the oestrogen receptor in lung immune cells and airway smooth muscle cell proliferation.42 Studies in mouse models of asthma have also shown that male and female hormones can affect mechanisms of inflammation involving macrophage polarisation.43,44 One study found that female lungs harbour greater numbers of type 2 innate lymphoid cells and more specifically a major subset of these cells lacking a killer-cell lectin like receptor G1, a population largely absent in male lungs and able to produce type 2 cytokines after sexual maturity.45 Experiments in gonadectomised mice with hormone replacement and mice bearing either global or lymphocyte-restricted oestrogen receptor α deficiency showed that androgens rather than oestrogens regulate the number of killer-cell lectin-like receptor G1 type 2 innate lymphoid cells subset and their functional capacity.45 Other studies have shown that Th2-mediated airway inflammation is increased by oestrogen and decreased by testosterone, and that females have increased IL-17A-mediated airway inflammation and increased dendritic cell and alveolar macrophage numbers and function when compared to males.46 Finally, studies in human cells obtained from asthmatic patients have found the effects of oestrogen to be mediated by oestrogen receptor β activation in airway smooth muscle proliferation and signalling pathways, which may point to a novel potential target for blunting airway remodeling.47 Together, these studies indicate that changes in oestrogen and androgen circulating levels might be the reason why asthma is more prevalent in females after reaching puberty. Overall, ovarian hormones have been found to increase, and testosterone to decrease, airway inflammation in asthma, but the mechanisms remain unclear.
When the effects of circulating sex hormone levels were studied in asthmatic children aged 6–18 years, both beneficial effects of androgens on lung function and symptom control, and weak deleterious effects of oestradiol on lung function, were observed.48 Starting at puberty, asthma symptoms increase in girls compared to boys, and fluctuations in hormones during menstruation are associated with changes in asthma symptoms.49 After puberty, sex hormones have also been found to regulate asthma symptoms during menstruation, pregnancy, and menopause,49 but studies on this topic are inconsistent and the mechanisms involved are not as clear. With regard to sensitisation and allergic responses, a recent review on immunological processes associated with IgE sensitisation concluded that female sex hormones are more likely to enhance immunological responses and exaggerated disease, whereas male hormones tend to dampen the same responses, thus increasing the risk of allergic diseases in adult females. However, these studies are controversial because female patients have also been shown to have lower IgE levels and sensitisation rates than males.50 The observed discrepancies among studies, particularly between the results of epidemiological and experimental studies, strongly suggest that more research needs to be done to reveal the mechanisms by which sex hormones contribute to allergy and asthma in children, adolescents, and adults.
CONCLUSION
It is clear from the studies presented above that differences exist in risk factors, incidence, and progression of asthma in the paediatric and adult population. In children, challenges remain in diagnostic strategies, particularly those associated with parental education to detect symptoms. In adults, environmental exposures, socioeconomic factors, and disease comorbidities have presented challenges establishing associations that can help identify asthma risks factors. In addition, the observed sex differences in asthma incidence and severity throughout life, with asthma affecting more boys than girls before puberty, and more women than men in the reproductive years, suggest that sex hormones play important roles in the development and progression of asthma. To study the specific effects of hormones in the paediatric and adult populations, studies in children and adolescents have been conducted to address the switch in the asthma prevalence ratio but have presented with conflicting results and identified comorbidities, such as obesity and allergy, as well as environmental and genetic factors. In adults, experimental evidence from human cells and animal studies have revealed pro-inflammatory effects of oestrogen and anti-inflammatory actions of androgens. Additional findings in women presenting with variable asthma symptoms throughout the menstrual cycle have supported the hypothesis that circulating oestrogen levels influence asthma in women. Despite this evidence, the specific mechanisms of action for sex hormones to promote or prevent asthma remain unclear. More research aimed at understanding the contributions of male and female hormones, and their receptors and signalling pathways, in both lung immune and structural cells may help to identify therapeutic targets that can be used to treat asthma in male and female patients at different moments of their reproductive life.








