Abstract
This narrative review aims to appraise the current perspectives on the diagnosis and treatment of asthma in childhood, with a focus on diagnostic steps, disease phenotypes and endotypes, and novel biologic therapies. Asthma in children and adults is now regarded as a complex cluster of disease phenotypes linked to specific endotypes. Unravelling asthma heterogeneity is key to understanding the pathogenic mechanisms of the disease and developing novel treatment strategies that are tailored according to these phenotypes and endotypes. This will make for a more precise diagnosis and more personalised treatments. There is currently no gold-standard method for making the diagnosis of asthma due to the non-specific nature of asthma symptoms; respiratory symptoms and airflow limitation need to be carefully evaluated to establish a causal relationship with the disease. Although corticosteroids and bronchodilators still constitute the recommended step-wise pharmacological based therapy in both childhood and adult asthma, novel biologic therapies targeting type 2 immunity have been proven effective in severe childhood and adult asthma and will likely lead to improved disease outcomes.
INTRODUCTION
Asthma is currently characterised, in functional terms, as an inflammatory disorder linked to airway hyper-responsiveness that leads to symptoms.1 The disease has thus been defined as a chronic inflammatory disorder of the airways involving many cells and cellular elements, which are associated with airway hyper-responsiveness leading to non-specific symptoms, such as recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, especially at night or in the early morning. These episodes (or exacerbations) are usually associated with widespread, but variable, airflow obstruction within the lung, which is reversible either spontaneously or with treatment.2 Asthma is one of the leading non-communicable respiratory diseases in children globally.3,4 In the UK,5 the USA,6 and China,7 asthma is reported to be a major cause of childhood morbidity with rising prevalence rates seen in developing countries, including China.7 Although the reason for this trend is not clear, the environmental pollution arising from industrialisation in these countries has been suggested as a major risk factor,7 especially considering that the disease occurs following a genetically driven abnormal response of the immune system to environmental air-borne allergens.8 However, both genetic (atopic) and non-genetic (non-atopic) asthma forms do exist: the latter being the more common form of presentation in adult patients.9
In children and adults, asthma is a heterogeneous disorder in which the asthmatic phenotype, including its clinical features, and the underlying endotype are complex and represent the myriad of host (gene)–environment interactions, which take place over different spatial scales and timescales.10 The heterogeneity of the disease is particularly evident following clinical presentation, as well as in the nature and severity of airway inflammation and remodelling. Thus, asthma is no longer regarded as a single-disease entity, but rather as a complex cluster of disease phenotypes.8
Multiple phenotypes have now been identified, which are based on different cellular and molecular mechanisms from the heterogeneity of its immunology.10 For instance, major inflammatory phenotypes, such as eosinophilic, neutrophilic, mixed complex inflammation, and pauci-granulocytic phenotypes, have been described from sputum cytological examination.11 Further discoveries have been made that have identified molecular phenotypes in keeping with high type 2 T cell immunity and low type 1 T cell immunity asthma.12 At present, a better understanding of the mechanisms of asthma immunopathology has led to the classification of the disease as either eosinophilic asthma (allergic and non-allergic), non-eosinophilic asthma (pauci-granulocytic and neutrophilic type 1 and type 17 T cell), or mixed granulocytic asthma.10
Unravelling the heterogeneity of asthma is a prerequisite for appreciating the pathogenic mechanisms of the disease and developing novel treatment strategies that are tailored according to phenotypes and endotypes. This will make for a more precise diagnosis and the provision of more personalised treatments. Although corticosteroids and bronchodilators still constitute the recommended step-wise pharmacologic agents in both childhood and adult asthma,13 novel biologic therapies targeting type 2 T cell immunity have undergone trials and have been proven to be effective in severe childhood and adult asthma.14,15
This narrative review aims to appraise the current perspectives on the diagnosis and treatment of asthma in childhood. Specifically, the review discusses the progress made so far in the phenotyping and endotyping of the disease, as well as the emergence of novel biologic and other therapies.
LITERATURE SEARCH STRATEGY
The PubMed database was searched for original research and review articles published within the last 20 years, to the day the search was carried out (10th September 2018). The search was conducted by combining the key term ‘asthma’ with each of the following descriptors: ‘childhood’, ‘diagnosis’, ‘spirometry’, ‘peak expiratory flow rate’, ‘phenotypes’, ‘endotypes’, ‘type 2 immunity’, ‘type 1 immunity’, ‘biologic therapy’, and ‘pharmacologic therapy’. Articles were selected in line with the specific objectives of the present review. The selected review articles consisted of both narrative and systematic reviews. Duplicated papers were excluded, while the remaining articles formed the bulk of the published literature used to produce this review.
ASTHMA DIAGNOSIS: ASSESSING SYMPTOMS AND AIRFLOW LIMITATION
Assessment of pulmonary function should complement symptom evaluation in the diagnosis of obstructive lung diseases, such as asthma.16 In fact, there is no gold-standard method for making a diagnosis of asthma; the symptoms and variability of airflow limitation are the parameters often evaluated during diagnosis.10 As a result of the non-specific nature of asthma symptoms, respiratory symptoms need to be carefully evaluated to establish a causal relationship between the presented phenotype and the disease.
Spirometry is a useful tool for assessing air flow limitation, especially in older children (aged ≥6 years) and adults: populations that can easily understand and co-operate with the procedure.17 While it is not commonly used in younger children, some preschool children have been shown to undergo acceptable spirometry as well.18 The procedure provides an objective evaluation of airflow limitation and reversibility, which makes it a useful tool for measuring bronchial responsiveness in suspected cases of asthma, as well as in diagnosing and differentiating obstructive from restrictive lung diseases.19 Using spirometry involves the correct interpretation of the values of forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and the ratio of FEV1 to FVC obtained from the spirometer.20 Asthma and other obstructive lung diseases are associated with a reduction in FEV1 relative to FVC and consequently a low FEV1:FVC ratio. Nevertheless, spirometry is limited as an evaluation tool in asthma. For instance, many intermittent and mild or stable asthmatics may have normal FEV1 and FVC values between acute exacerbations. Thus, the procedure is more useful as a monitoring tool since a sudden decline in FEV1 or other measures in the same patient can signal poor asthma control, even with normal baseline values.
While limited, spirometry is useful for the assessment of air limitation reversibility after the administration of a bronchodilator. The American Thoracic Society (ATS) and the European Respiratory Society (ERS) define this reversibility as an increase in FEV1 of ≥12% from baseline and an absolute FEV1 increase of ≥200 mL; an elevation of FVC by ≥200 mL also constitutes reversibility.21 It has been suggested that postbronchodilator spirometry should not be done in all patients with normal baseline values unless asthma is strongly suspected on clinical grounds.22 For instance, a recent study, which reported a postbronchodilator improvement in 3.0% of 1,394 patients with normal baseline results,23 further corroborates this recommendation,22 while also highlighting the poor sensitivity of FEV1 as a variable in evaluating the disease.
When spirometry fails to demonstrate significant reversibility of airflow limitation and clinical suspicion of asthma remains strong, broncho-provocation testing (usually with methacholine) is recommended.24 Despite the predictive ability of a positive methacholine-challenge test for asthma in patients with atypical symptoms or normal baseline values, false-positive results are also obtainable. The high negative predictive value of broncho-provocation testing with methacholine makes it a useful test for excluding the diagnosis of asthma, since negative results are rarely false-negatives.24 Adequate standardisation of this test is vital to enable the best differentiation between normal airway responsiveness and airway hyper-responsiveness, as well as to compare results between different methods.25 Thus, standardisation documents have recently been updated by the ERS to achieve these objectives.25 It is also important to note that pharmacologic agents may inhibit airway responsiveness to methacholine through one of the following mechanisms: specific antagonism (e.g., antimuscarinic agents), functional antagonism (e.g., other bronchodilators, especially β agonists), or an anti-inflammatory effect (e.g., corticosteroids).25 An understanding of the effects of these pharmacologic agents on the outcome of the methacholine challenge test is therefore essential as it will aid in guiding the appropriate washout periods from therapeutic interventions that inhibit airway responsiveness: an important aspect of the standardisation.25
Spirometry is also useful for the assessment of disease severity and reversibility of airflow in order to guide therapy. Generally, asthma symptoms do not correlate with disease severity. Asthma symptoms poorly reflect airflow reversibility and may lead to inaccurate assessment of the degree of airflow limitation.26 This disparity between symptoms and disease severity and airway reversibility is confirmed by the findings of a study that showed that the majority of the evaluated asthma patients had FEV1 and FEV1/FVC values that were not directly related to self-reported disease exacerbation or subjective measures of disease severity or treatment response.27 In comparison to the assessment of asthma symptoms, spirometry provides a more objective approach to assess asthma severity and response to treatment. The Global Initiative for Asthma (GINA) guidelines recommend the use of spirometry every 1–2 years or more, and at the initial evaluation, after treatment is initiated and symptoms are under control, and during periods of progressive deterioration or improvement of symptoms.28 In resource-poor settings, where spirometers are not readily available, measurement of peak expiratory flow rate using the peak flow metre may be an acceptable alternative.20 Airway constriction leads to lower peak flow readings. Changes in recorded values can be used to determine lung function, the severity of asthma symptoms, and therapy. Some asthma patients may benefit from regular peak flow monitoring, especially if the process is conducted simultaneously with the review of asthma symptoms and the frequency of reliever medication use. The measurement of peak expiratory flow rate requires training in the correct use of the peak flow meter while values are affected by confounders such as the patient’s sex, age, and height. Thus, the wide range of ‘normal’ values makes it a less recommended test to evaluate asthma.
Finally, spirometry may also be useful for predicting asthma exacerbations. For instance, FEV1 values, in comparison with parent-completed or patient-completed questionnaires, were reported to have a strong association with the risk of asthma exacerbations in children.29 In summary, spirometry remains an important objective tool to measure lung function, which facilitates asthma diagnosis when used together with symptom evaluation. It should be performed in all patients in whom asthma is suspected, both at the point of diagnosis and subsequently at intervals, to evaluate disease progression.
ASTHMA PHENOTYPES, ENDOTYPES, AND BIOMARKERS
Although childhood asthma has previously been classified into two main phenotypes, namely allergic asthma and non-allergic asthma,30 several novel phenotypes have now been identified from latent-class and cluster analyses,31-36 with considerable overlaps of clinical features in phenotypic cluster groups.31 During early childhood, the onset and pattern of wheezing over time has often been used to define asthma phenotypes, whereas in older children variables like atopic status and risks, decline in pulmonary function, or exacerbation risks have been incorporated into the designation of phenotypes, making prediction of treatment outcome possible.37
Taking several factors into consideration, diverse phenotypes of childhood asthma have been described by many studies. For instance, two prospective cohort studies, which evaluated long-term outcomes of early childhood phenotypes, have identified phenotypes based on asthma onset,33 and patterns of wheezing.34 In the BAMSE cohort study outcomes at age 8 and 16 years,33 phenotypes were classified into never asthma, early transient asthma, early persistent asthma, and late-onset asthma. Decline in pulmonary function typically occurred in all the designated phenotypes except the never asthma phenotype. The Melbourne Atopy Cohort Study34 placed children into five groups (never/infrequent wheezers, early transient wheezers, early persistent wheezers, intermediate-onset wheezers, and late-onset wheezers) based on the pattern of wheezing at 12 and 18 years of age. The persistent wheeze phenotype was characterised by reduced growth in prebronchodilator FEV1 over adolescence, with the intermediate-onset wheezers showing irreversible airflow limitation by 18 years of age.
Furthermore, classification of childhood asthma phenotypes via cluster-analysis study has been conducted.31 This study, from the Childhood Asthma Management Program (CAMP), defined five cluster phenotypes on the basis of atopy burden, airflow limitation, and history of exacerbation. In cluster 1, the phenotype is described as mild asthma with low atopy, obstruction, and exacerbation rate; in cluster 2, the phenotype is atopic asthma with low levels of obstruction, and medium rates of exacerbation; cluster 3 refers to atopic asthma with high levels of obstruction, and medium rates of exacerbation; in cluster 4, moderately atopic asthma with high levels of obstruction and high exacerbation rates is the identified phenotype; while cluster 5 describes highly atopic asthma with high levels of obstruction and high exacerbation rates.31 The prediction of bronchodilator response can easily be made from these phenotypic clusters: cluster 1 shows the lowest response while cluster 5 is characterised by highest bronchodilator response. Interestingly, heterogeneity in therapeutic response to inhaled steroids versus placebo or nedocromil (a mast cell stabiliser) was also observed between the phenotypic groups.31 For instance, during asthma exacerbations, clusters 1, 2, and 3 showed better response to inhaled steroids compared with the administration of nedocromil or the placebo, whereas cluster 4 responded better to either steroids or nedocromil than to placebo; but there were no differences in the responses to these therapeutic interventions by cluster 5.31
To underscore the current diversity in phenotyping childhood asthma, it is important to mention other notable classifications. In the MeDALL project, Garcia-Aymerich et al.32 studied large populations of children at 4 and 8 years of age from seven European population-based birth cohorts using latent class and cluster analyses. Researchers concluded that allergy-related diseases in children (asthma, rhinitis, and eczema) are more accurately grouped together as an allergic comorbidity cluster, than as three distinct diseases. Thus, this cluster consists of phenotypes, including asthma only, asthma and allergic rhinitis, asthma and atopic dermatitis, and asthma with atopic dermatitis and allergic rhinitis. Interestingly, a decline in pulmonary function from birth to 16 years was observed in the asthma with atopic dermatitis and allergic rhinitis phenotype but not in the other phenotypes.38 Further classification of severe childhood asthma into sub-phenotypes reveal differences compared with severe asthma in adulthood.37,39 For instance, eosinophilic airway inflammation occurs in both childhood and adult severe asthma. Male predominance and severe atopy are the hallmarks of severe asthma in children, whereas adults with severe asthma are predominantly non-atopic females with aspirin sensitivity and nasal polyposis.39 Additionally, both children and adults have airway remodelling, but this is worse in adults with severe asthma who also exhibit fixed airway obstruction. In a prospective Brazilian study,40 a therapy-resistant asthma sub-phenotype was described in children with severe asthma, characterised by low FEV1 and high fractional exhaled nitric oxide (FeNO). Similarly, in adults with severe asthma, a sub-phenotype identified as frequent exacerbators, also showed high FeNO, but these individuals had a history of smoking.41 Despite the abundance of work, more research is required to link these phenotypes with underlying immunopathology and genetics.
Given that asthma is a heterogeneous and genetically complex disease, it is likely to have several specific endotypes linked to distinct clinical characteristics, different underlying molecular aetiologies, and distinct therapeutic responses.1 Progress in understanding the pathophysiological mechanisms of the disease have resulted in the identification of biologically distinct variants.42 The definition of asthma endotypes will lead to precision in disease classification and the identification of biomarkers that fulfil conventional diagnostic or prognostic criteria.1 Furthermore, grouping patients into endotypes will enable effective preventive measures and identification of appropriate individualised treatments.
The traditional concept of clinical asthma is predicated by the Th2 inflammation hypothesis. It is believed that exposure to allergens propels eosinophilic inflammation and tissue injury resulting in airway hyper-responsiveness and mediator release, which are responsible for asthma symptoms.1 Thus, two distinct endotypes have been recognised: Th2-high and Th2-low asthma. Classification depends on the expression of the Th2 cytokines, including genes encoding for IL-4, IL-5, IL-9, and IL-13, as well as the treatment response to inhaled corticosteroids (ICS).43
Although the Th2 inflammation hypothesis presents a molecular framework underpinning the associations of atopy or IgE and eosinophilic-linked lung inflammation with asthma, the hypothesis is now being challenged on the basis of some observed discrepancies.1 For instance, the Th2 inflammation model predicts that eosinophilic inflammation should propel airway hyper-responsiveness, but no obvious relationship has been observed,44,45 and there is also non-concordance between atopy and airway hyper-responsiveness.46 Consequently, a new model for childhood asthma endotype has been proposed. In this new model, distinct endotypes arise through variable interplay of the components: asthma symptoms frequently result from the interaction between altered lung function (FEV1, airway hyper-responsiveness, and airway smooth muscle) and immunopathologies (airway remodelling and inflammation), while modifying processes (BMI, diet, and in utero exposures), acute asthma exacerbations, cigarette smoke, and environmental irritants impinge on lung function and remodelling and inflammation.1
Although the classification of endotypes in childhood asthma is still developing, the presence or absence of a Th2-molecular imprint has therefore enabled the linkage of asthma endotypes with phenotypes in order to identify patients who would respond or fail to respond to treatment with ICS.47,48 As recent updates shed more light on asthmatic pathogenesis, disease classification has now been able to link phenotypes to endotypes based on airway and serum biomarkers, for example, specific IgE, Th2 cytokines (early-onset allergic phenotype); eosinophilia, IL-5 (late-onset eosinophilic phenotype); sputum neutrophilia, IL-8 (neutrophilic phenotype); and FeNO (smoking- induced phenotype).47 A potential nexus exists between observable disease characteristics (phenotype) and underlying pathobiology (endotype) or biomarkers, as well as some potential personalised therapeutic options. In the future, endotypes may be used together with specific biomarkers to predict responses to targeted therapy.47,49 For instance, Cowan et al.50 reported that a noninvasive panel of inflammatory biomarkers in steroid-naïve asthmatics predicted clinical responsiveness to ICS.50 The authors specifically measured FeNO values, sputum eosinophil counts, and urinary bromotyrosine levels. Notably, the combination of high FeNO values and high urinary bromotyrosine levels had the best predictive ability for a favourable response to ICS.50 Urinary bromotyrosine is a noninvasive biomarker of eosinophil-catalysed protein oxidation, which has been shown to measure asthma control and predict disease exacerbations in children,51 while FeNO is considered a biomarker of Th2 inflammation and atopy rather than a marker for evaluating severe asthma.52 Currently, FeNO is seen as a useful marker of asthma severity, recognising specific asthma phenotypes (such as allergic asthma in childhood and smoking-induced asthma common in adults), predicting efficacy of standard corticosteroid and biologic therapies, as well as evaluating patients with allergic rhinitis at risk of asthma.53 A recent retrospective study further confirms the ability of FeNO (in combination with blood eosinophil count) to identify patients with frequent asthma exacerbations and to stratify the appropriate therapy for Th2 inflammation-predominant severe asthma.54 Blood eosinophil count is considered the best evaluated biomarker, the most applied in clinical practice, and, until recently, the most promising of all blood biomarkers.55 Aside from blood eosinophil count, using other blood biomarkers for asthma diagnosis has not been encouraging. It may be trite to recall that the major biomarkers used in investigating the disease are generally present either in the sputum (e.g., sputum eosinophils and neutrophils), exhaled breath (e.g., FeNO), or in blood (e.g., blood eosinophils, IgE, and periostin). Several Th2-high asthma biomarkers have been extensively evaluated and validated: especially FeNO, blood eosinophil count, and serum periostin.56 However, there is still a lack of biomarkers for Th2-low asthma. Therefore, characterising the Th2-low endotype essentially requires the absence of any known biomarkers of Th2-high asthma, as some investigators recently used these cut-off points (IgE ≥100 IU/mL, blood eosinophil count ≥300/μL, and FeNO ≥30 ppb) to define high levels of Th2 immune activation: grouping patients as having either a high Th2 immune profile (≥2 raised Th2 biomarkers) or a low Th2 immune profile (≤1 Th2 biomarkers).57 This method appears logical until a validated Th2-low biomarker that can be used in clinical practice is discovered.
PHENOTYPE AND ENDOTYPE-DRIVEN (BIOLOGIC) THERAPIES
Novel treatments, which are based on disease pathobiology, may offer a more effective option for managing severe asthma in patients who do not respond to conventional therapies such as corticosteroids and bronchodilators. These patients have an elevated airway eosinophil count which is characteristic of Th2 inflammation.
Biologic therapies that target the Th2 signalling pathway and eosinophils constitute a paradigm shift in the treatment of severe asthma.58 Examples of these treatment options include the anti-IL-5, anti-IL5R, and anti-IgE therapies. Most of these biologic therapies have undergone successful Phase III trials in adult asthma,59-61 and in both adult and childhood asthma (Table 1).14,15,62-67 Firstly, omalizumab (an anti-IgE), the first monoclonal antibody therapy for severe asthma, is currently licensed for moderate-to-severe allergic asthma in adults and children (>6 years) with IgE >30 IU/L.10 Omalizumab has been observed to reduce exacerbations and hospital admissions in adults and children,63 while another study showed that the therapeutic response was predicted by elevated high Th2 immunity biomarkers (namely high FeNO, serum periostin, and blood eosinophils) rather than IgE concentration.64 It also reportedly reduced virus-associated exacerbations.65,66 The drug particularly improved IFN-α responses to rhinovirus in school children.66 Recently, omalizumab has been added to Step 5 of treatment in the latest GINA recommendations, which include other therapeutic options like tiotropium and oral corticosteroids.13 It has been noted that omalizumab is more likely to be useful in patients exhibiting high levels of Th2 inflammatory biomarkers, such as FeNO, blood eosinophil count, and serum periostin,68 which corroborates the findings of the previous study.64
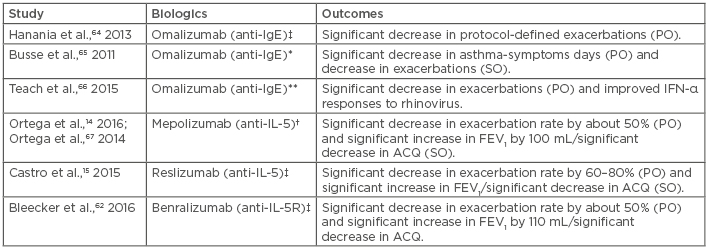
Table 1: Novel biologic therapies currently found to be effective in moderate-to-severe asthma in childhood.
†Trialled in adults and children (>12 years); ‡Trialled in adults and children (12-75 years); *Trialled in children, adolescents, and young adults (6-20 years); **Trialled in school children (6-17 years).
ACQ: asthma control questionnaire; FEV1: forced expiratory volume in first second; IL-5R: interleukin 5 receptor; PO: primary outcomes; SO: secondary outcomes.
Secondly, mepolizumab (anti-IL-5) has been found to significantly reduce exacerbation rate by 50% as well as increase the FEV1 in adults and children (>12 years) with moderate-to-severe asthma.14 Another anti-IL-5 monoclonal antibody which has been trialled in childhood and adult moderate-to-severe asthma is reslizumab; the drug was also noted to have significantly reduced the exacerbation rates and increased the FEV1.15 Finally, treatment with benralizumab (anti-IL5R) was found to have resulted in similar primary and secondary outcomes with a significant reduction in exacerbation rates and increase in FEV1.62
OTHER CURRENT TREATMENT OPTIONS
Despite the prospects biologic therapies hold for personalised treatment and improved outcomes in children with moderate-to-severe asthma, the traditional pharmacologic therapies with bronchodilators and corticosteroids remain effective. The step-wise pharmacologic treatment with these classes of drugs as recommended by GINA is still the current practice.13 If symptoms and exacerbations are not controlled, drug and dose adjustments are made as per the steps.
In this treatment approach for adults, adolescents, and children (≥6 years), the Step 1- recommended drug for all ages is the inhaled short-acting β agonist (e.g., salbutamol or terbutaline) acting as reliever medication. Alternatively, a regular low-dose ICS is added to reduce asthma symptoms, exercise-induced broncho-constriction and exacerbation risk, and halt the attendant decline in pulmonary function. For Step 2, regular low-dose ICS is recommended, with as-needed short-acting β agonist for all ages: another option being regular low-dose ICS and long-acting β agonist (LABA) for adults and adolescents. In Step 3, low-dose ICS and LABA are recommended for adults and adolescents. ICS and maintenance and reliever therapy are recommended for the same age groups in Step 4, while referral for assessment is advised for children. Referral for further assessment is recommended in Step 5.
Another currently proposed treatment option for asthma are the macrolide family of antibiotics. The beneficial effects of macrolides in asthma are thought to be related to either their anti-inflammatory action and/or their inhibitory action on the airway virobiome and microbiome.69 For instance, low-dose azithromycin reduced the rate of exacerbations in patients with noneosinophilic asthma.70 Furthermore, as an add-on treatment to medium-to-high-dose ICS plus LABA, it resulted in reduced exacerbation rate and quality of life improvements in patients with uncontrolled, persistent asthma.71 Recently, Stokholm et al.72 reported in their randomised, double-blind, placebo-controlled trial that azithromycin alone reduced the duration of episodes of asthma-like symptoms in children aged 1–3 years, and also suggested that this antibiotic potentially has a role in acute management of exacerbations.72 However, there is a cautious optimism about its use because of the risk of microbial resistance, as well as the limited evidence about its role in severe asthma.
Another group of medications currently used in mild or severe asthma in children is the leukotriene modifiers.73 Leukotrienes (LT), namely cysteinyl LT (CysLT) and LTB4, are potent lipid mediators that play an important role in the pathophysiology of asthma phenotypes: with two identified receptor subtypes for CysLT (CysLT1 and CysLT2), activation of CysLT1 receptor results in increased airway smooth muscle activity, microvascular permeability, and airway mucus secretion, while LTB4 may be involved in the development of airway hyper-responsiveness, severe asthma, and asthma exacerbations.73 Despite the less effective action of CysLT1 receptor antagonists such as montelukast, pranlukast, and zafirlukast when compared to ICS, they can either be administered orally as monotherapy in patients with persistent mild asthma or in combination with ICS in patients with more severe asthma.
CONCLUSIONS
The current concept regarding asthma is that of a heterogeneous and genetically complex disease with several phenotypes having unique clinical characteristics linked to endotypes with different underlying mechanisms and distinct responses to treatment. Better understanding of the pathophysiologic mechanisms of the disease has resulted in the identification of these endotypes. Definitions of asthma endotypes will lead to precision in classifying disease and identifying biomarkers that meet formal diagnostic or prognostic criteria, as well as enable identification of appropriate individualised treatments. Thus, biologic therapies which may in future represent a paradigm shift from the traditional pharmacologic therapies will lead to improved disease outcomes in moderate-to-severe childhood asthma (in which they are currently indicated). However, the drawbacks to their routine use in children include their parenteral form and high cost of treatment.








