BACKGROUND AND AIMS
Before 2021 in Calgary, Canada, hospitalized inpatient latent tuberculosis infection (LTBI) screening, with a QuantiFERON assay (QFT), for immunosuppression, required approval from a tuberculosis physician. This may have delayed testing, thereby resulting in QFT collection after immunosuppression initiation. An inpatient QFT order set was implemented in November 2020, permitting screening without approval for patients tested due to immunosuppression. The impact of the order set on local LTBI screening practices is yet to be investigated. Additionally, there are limited published data on inpatient QFT testing. The study’s objectives were to assess the impact of the order set on the timing of QFT collection in relation to immunosuppression initiation; describe characteristics of patients undergoing QFT testing and their test results; and assess the relationship between administration of inpatient immunosuppression and QFT results.
MATERIALS AND METHODS
A cross-sectional analysis was completed with a chart review of all adult inpatients who underwent a QFT at four acute care hospitals in Calgary, Canada, from January 1–October 31, 2020, and January 1–December 31, 2021. November 1–December 31, 2020 were excluded due to the roll-out of the order set. Z test was used to assess the proportion of pre-immunosuppression QFT testing pre- and post-order set implementation; associations were analyzed using logistic regression.
RESULTS
A total of 639 inpatients had QFT testing, of which 534 (191 pre-order set, 343 post-order set implementation) were for immunosuppression screening. Of those tested due to immunosuppression, there was a significant decrease in the initiation of inpatient immunosuppression before collection of QFT following implementation of the order set (103/191 [54%] versus 153/ 343 [45%] in 2020 and 2021, respectively [p=0.0388]). Among all inpatients, there were 34 positive (5%), 496 negative (87%), and 109 indeterminant (17%) QFT results. Indeterminate results were more frequent among those who were administered immunosuppression in-hospital before QFT testing (either new start of immunosuppression, new medication, and/or increased dose if taking home immunosuppression; 76/279 [27%]) compared to those without new, or escalation of, immunosuppression before QFT (33/360 [9%]; p=0.0004). Univariable logistic regression analysis of variables potentially associated with indeterminate results is shown in Table 1.
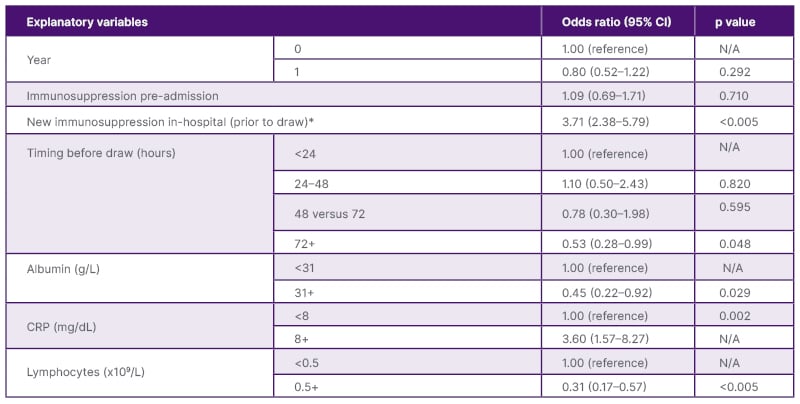
Table 1: Univariable logistic regression analysis of variables potentially associated with indeterminate results.
*Defined as either a new start of immunosuppression and/or increased dose administered before QFT collection.
CRP: c-reactive protein; N/A: not applicable; QFT: QuantiFERON assay.
CONCLUSION
To the authors’ knowledge, this is the largest review of in-hospital QFT results. Immunosuppression started in-hospital before QFT collection was associated with a high rate of indeterminate QFT, which limits its utility. Implementing an order set significantly reduced the proportion of QFT testing done after in-hospital immunosuppression initiation (i.e., reduced delay in testing). However, QFT collection still occurred frequently after initiation of immunosuppression. While beneficial, an order set alone may be insufficient to drastically alter practice patterns with regard to LTBI screening; a multimodal approach to this problem is required.







