INTRODUCTION
Progressive pulmonary fibrosis (PPF) is an increasingly recognised condition, defined in 2022 to address the progression of pulmonary fibrosis in patients with interstitial lung diseases (ILD) other than idiopathic pulmonary fibrosis (IPF).1 Oral pirfenidone has been studied in non-IPF ILDs but never achieved a statistically significant change in the primary endpoint. Trends seen in secondary endpoints support efficacy in PPF.2-4
OBJECTIVE
Data from the AP01-002 (ATLAS) Study of inhaled pirfenidone in IPF demonstrated efficacy and improved safety compared to that seen with oral pirfenidone.5 The AP01-007 (MIST Study, Figure 1) is designed to study the efficacy and safety of AP01 (aerosolised pirfenidone) in patients with PPF. Patients will remain on background immunosuppression, and up to 30% of patients will remain on background nintedanib therapy.
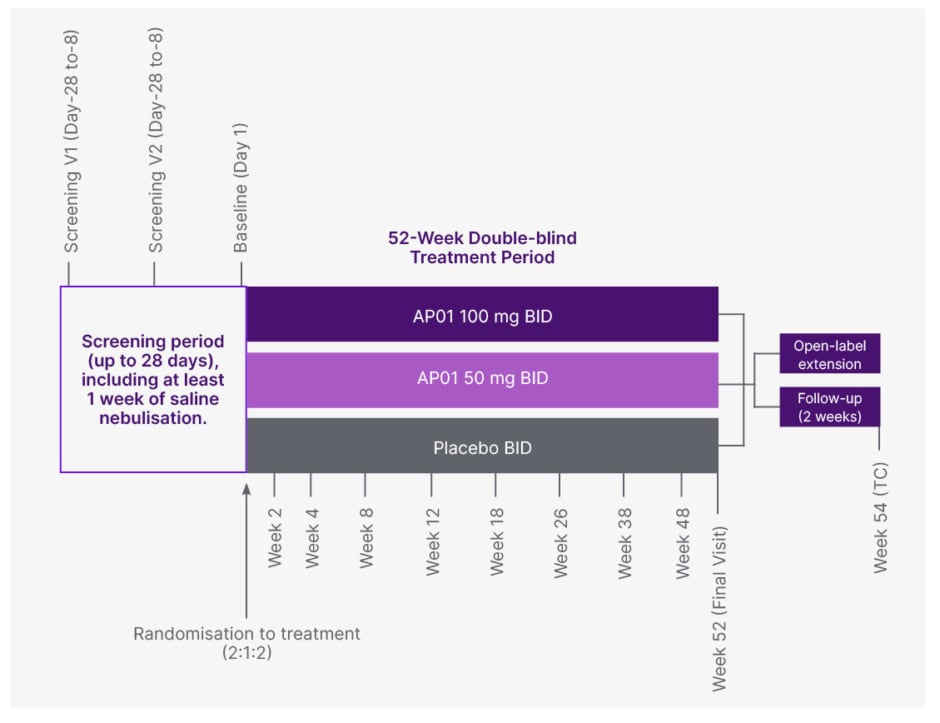
Figure 1: MIST study design.
BID: twice daily; TC: telephone call.
METHODS
The primary objective is to observe the change in the annual rate of decline in forced vital capacity in the AP01 treatment groups as compared to placebo. In addition, efficacy will be measured in secondary endpoints of time to progression, change in 6-minute walk test, change in fibrotic scores via quantitative high-resolution CT, and change from baseline quality of life. Safety outcomes will be assessed as well. Cough will be analysed through cough counts and cough questionnaires, which should allow differentiation of cough related to PPF versus the nebulisation procedure.
CONCLUSION
MIST will study the safety and efficacy of AP01 (aerosolised pirfenidone) in patients with PPF. In addition to the safety and efficacy endpoints, MIST will carefully examine the presence of cough in this population of patients.







