Meeting Summary
The landscape of assisted reproductive technology (ART) is shifting, with an increasing move toward frozen embryo transfers (FET). Multiple studies have confirmed the pivotal role of progesterone in the establishment and maintenance of pregnancy. However, challenges arise when applying progesterone supplementation protocols developed for endometrial preparation in fresh transfers to the new ‘freeze-all’ era. In particular, clinical questions surround the efficacy of vaginal progesterone in FETs, with one-third of patients failing to achieve sufficient levels of this key hormone using standard micronised vaginal progesterone (MVP) supplementation. In this symposium, leading experts in reproductive medicine considered alternative routes of progesterone administration and discussed the best strategy to rescue a cycle in cases of inadequate levels, with the overall aim of optimising reproductive outcomes after FET. In the new freeze-all era, self-administered subcutaneous (SC) progesterone injections offer a valid and efficient alternative to intramuscular (IM) and vaginal progesterone preparations for luteal-phase support in FET, which obviates the need for ongoing progesterone level monitoring. Emerging evidence indicates that a single SC progesterone injection (25 mg/day) can effectively rescue the cycle at any point during the luteal phase, and twice-daily injection is equivalent to IM progesterone (50 mg/day) in priming for FETs.
New Challenges of the Freeze-All Era
Annalisa Racca
The last decade has seen a sharp increase in the number of freeze-all cycles and subsequent FETs. Annalisa Racca outlined the challenges and opportunities presented by the freeze-all era, focusing on the key role of progesterone assessment and supplementation to maximise FET cycle success. ‘One size fits all’ no longer applies in the freeze-all era, explained Racca; instead, each female needs to be managed individually in order to optimise reproductive outcomes.
Improvements in laboratory techniques over recent years have led to a considerable rise in the number of supernumerary embryos being frozen after a fresh cycle. There has also been a sharp upsurge in the number of freeze-all cycles carried out for both elective and non-elective reasons (e.g., the COVID-19 pandemic). The net result is that ever-increasing numbers of FETs are being performed. Figures from a UK registry revealed a 707% increase in the number of freeze-all cycles carried out over the 5-year period to 2018. A total of 7,031 freeze-all cycles were carried out in 2018, compared to just 871 in 2013.1
The key advantage of the freeze-all era is that it allows reproductive medicine to be viewed not just in the context of a single child, but as an overall family project. However, every big change that happens in medicine also brings significant challenges, noted Racca. With the freeze-all era, these challenges lie in the need to adapt and develop better strategies in order to achieve the same or higher pregnancy and live birth outcomes with FET as with fresh embryo transfers. FET can be performed in a natural cycle, in a modified natural cycle where ovulation is induced with human chorionic gonadotropin (hCG), or in an artificial cycle, the so-called hormone replacement therapy (HRT) cycle. No one approach has been demonstrated to be superior to the other, as all have specific pros and cons.
The HRT cycle using an artificially prepared endometrium is the most widely used worldwide, and benefits from minimal cycle monitoring and easy scheduling. This approach can be applied equally to every single female at any age or stage in their reproductive journey, confirmed Racca. Disadvantages include cost, inconvenience, and the potential thrombotic side effects of estradiol (E2). The two main players in HRT cycles are the oestrogens and the progesterones. Studies looking at priming of the endometrium with E2 have shown no impact of length, dose, or serum level on cycle outcomes including clinical pregnancy rate and miscarriage rate.2-4 So, while oestrogens are obviously important, they are not as crucial in dictating cycle outcomes as progesterone, concluded Racca.
Progesterone plays several critical roles in human reproduction, facilitating transformation of the endometrium into a receptive environment and performing the dual functions of immunomodulation and myometrial quiescence, which are vital in reducing the risk of preterm deliveries. However, the optimal way to administer and measure progesterone in FETs remains a key question, said Racca. It is important to be aware that different administration routes for progesterone (vaginally, IM, or SC) will result in differing pharmacokinetic profiles. Studies have confirmed that serum concentrations of progesterone are higher with SC or IM administration, while endometrial concentrations are higher with vaginal dosing.5 Irrespective of the route of administration, significant variability in progesterone levels is also evident throughout the course of a day during both the luteal phase and the late follicular phases.6,7
When is best to assess progesterone during the luteal phase is another critical question in ART. In a study where progesterone assessment was performed the day before FET, a significantly lower live birth rate (47.5%) was observed in patients with a progesterone value <10.64 ng/mL compared to those with a progesterone level above this threshold (62.3%; p=0.017).8 Another study that measured progesterone on the day of FET found a lower pregnancy rate in patients with progesterone values <9.2 ng/mL (the pregnancy rate was 32.7% below this cut-off and 52.8% above it).9 A third study where progesterone was assessed 11–12 days after FET confirmed that progesterone levels <35 nmol/L, corresponding to approximately 10 ng/mL, were associated with a lower pregnancy rate (38% versus 51%, respectively).10
These data clearly demonstrate that, regardless of the timing of progesterone assessment, outcomes from the cycle are consistently improved with higher progesterone concentrations, stressed Racca. However, available evidence indicates that a substantial proportion of patients undergoing FET fail to achieve adequate levels of this essential hormone. One-third of those receiving MVP showed inadequate levels of progesterone in a recent 2020 study. Progesterone concentrations were found to be suboptimal (<8.8 ng/mL) in around one-third of cases across all the different cycles performed, including pre-implantation genetic testing for aneuploidy (PGT-A) or non-PGT-A with own oocytes and oocyte donation.11
Given the evidence that progesterone is a key driver of cycle outcomes, several studies have explored the important question of how and when to use progesterone supplementation to rescue a FET cycle. In a retrospective study of 227 FET cycles, inadequate serum progesterone levels <10 ng/mL were identified in 37% of cycles on embryo transfer day, after participants received 600 mg/day vaginal supplementation.12 In these people with suboptimal progesterone, MVP dose was increased to 1,200 mg/day and blood levels retested 2 days later. However, progesterone concentrations remained below the key 10 ng/mL threshold in 31% of women on re-evaluation, despite doubling of the dose of vaginal supplementation.12 Racca suggested that this could be due to malabsorption of vaginal progesterone, pharmacokinetics, saturation of the receptors, or the influence of the microbiota. It is not known why, she conceded, but it can be seen from this study that doubling the dose of vaginal progesterone is “not the ideal solution.”
A further retrospective study investigated combined supplementation with SC and vaginal progesterone from Day 1 of the luteal phase in 320 FET cycles conducted in 213 females.13 By using combined vaginal (800 mg/day) and SC (25 mg/day) doses, 95% achieved progesterone levels >10.5 ng/mL, with a minimum value of 7.2 ng/mL. Analysis of outcomes by progesterone quartiles revealed higher ongoing pregnancy rates (35.6% versus 26.3%) and lower miscarriage rates (12.3% versus 27.6%) in the upper two quartiles of serum progesterone (>21.95 ng/mL) compared to the lower quartiles.13
Racca went on to describe findings from a prospective observational study carried out by her own group, which investigated rescue of the FET protocol with daily SC progesterone injections in 574 HRT cycles (453 patients).14 In this study, serum progesterone was assessed the day before FET and, if found to be below the key threshold of 10.6 ng/mL, was supplemented from the day of embryo transfer with SC progesterone. Overall, 38% of women were found to have inadequate progesterone levels with standard vaginal supplementation, but >98% were able to reach levels >10.6 ng/mL with SC progesterone injections. Females who received daily SC progesterone injections started the day prior to FET achieved similar reproductive outcomes compared to those with initial adequate progesterone levels, with ‘excellent’ ongoing pregnancy and live birth rates (Figure 1).14 These findings show that there is a ‘window of opportunity’ where we can rescue the protocol and safeguard reproductive outcomes, even on the day of the FET itself, noted Racca.
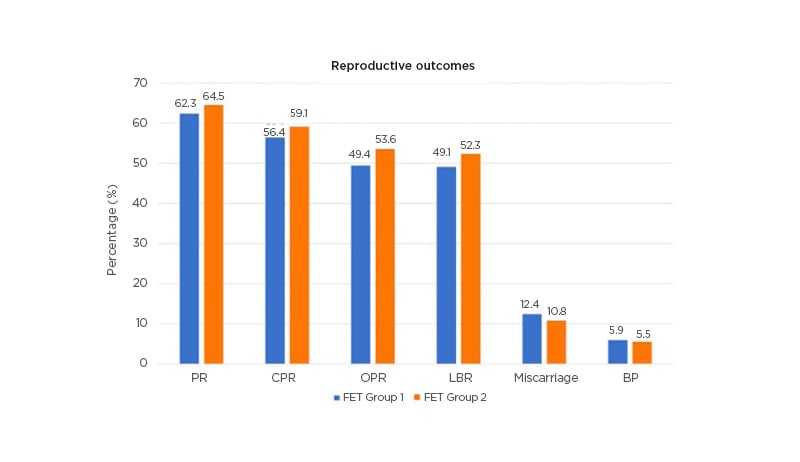
Figure 1: Subcutaneous progesterone injections can rescue the frozen embryo transfers protocol in cases of low progesterone.14
BP: biochemical pregnancy; CPR: clinical pregnancy rate; FET: frozen embryo transfer; LBR: live-birth rate; OPR: ongoing pregnancy rates; PR: pregnancy rate; SC: subcutaneous.
Other key issues to consider in the freeze-all era include whether single measurements of progesterone are sufficient or if repeated testing is more meaningful, and how late in the luteal phase a cycle can still be rescued. To answer these questions, Racca’s team conducted a further study in which progesterone assessments were performed both the day before FET and on the day of the hCG test itself.15 Results revealed that, even in the population of women with normal progesterone on the day prior to FET, 30% had dropped to inadequate progesterone levels by the day of the hCG. This fall in progesterone levels was evident regardless of the specific treatment they were undergoing (i.e., RECEP, PGT-A, or CT Propios). In this study, rescue of the protocol with SC progesterone even as late as the day of hCG in women with inadequate progesterone levels was still able to save the cycle.15 Racca described these findings as ‘striking’, with miscarriage rates consistently above 60% in the women with progesterone levels <10.6 ng/mL who did not receive supplementation, compared to low single-digit miscarriage rates in those whose cycles were rescued with SC progesterone.15
Overall, this collective clinical evidence confirms that luteal progesterone is a very strong predictor of cycle outcomes in FET HRT. Yet one-third of females receiving standard MVP supplementation still show inadequate levels of progesterone in any of their luteal measurements. Individualisation of progesterone supplementation in FET HRT and application of rescue protocols, such as SC progesterone injections, are therefore vitally important, concluded Racca, and can be implemented at any point during the luteal phase
Tomorrow’s Perspectives for Endometrial Preparation
Dominique de Ziegler
In this talk, Dominique de Zeigler outlined key problems that have been encountered in the increased shift towards frozen from fresh embryo transfers and shared potential solutions for adapting existing FET regimens to optimise endometrial preparation moving forward, based on available clinical evidence. None of the progesterone preparations currently available and in clinical use have ever been formally tested in FET, stressed de Zeigler. Ovarian progesterone production also shows a progressive increase during the early weeks of pregnancy, which must be compensated for during FET.16
There is a clear contrast in the underlying hormone profiles encountered in fresh versus frozen ART. In fresh embryo transfers, the key issue is decreased production of progesterone during the luteal phase arising from the impact of treatment on the pituitary gland. However, as soon as the patient becomes pregnant, normal progesterone production by the corpus luteum resumes under activation by hCG. The progesterone support provided usually continues until the luteo-placental shift, although de Zeigler emphasised that this is not really necessary as, in fresh cycles, progesterone can actually be stopped on the day of the positive pregnancy test itself without detrimental consequences.
However, in FET, the situation is completely different because there is no endogenous hormone production, explained de Zeigler, which must be supplied in the form of E2 and progesterone supplementation regimens. Progesterone supplementation is required to cover not only the luteal phase, but also the increased production that occurs in the early weeks of the pregnancy. The amount of progesterone delivered therefore has to be increased, stressed de Zeigler, and while 25 mg/day is sufficient in fresh cycles, a higher dose, such as 50 mg/day, may be required to achieve optimal outcomes in FET. Failure to achieve adequate levels of progesterone has detrimental consequences, he added.
Although vaginal progesterone has been used for many years and is approved for fresh embryo transfers, failure to achieve adequate blood levels when used in FET is a common clinical problem. This shortcoming in vaginal progesterone regimens may require rescue options to be initiated if progesterone levels in a FET cycle fall too low. As illustrated by Racca, evidence shows that lower quartiles of progesterone on the day of embryo transfer, notably serum levels <9.2 ng/mL, are associated with worse reproductive outcomes, including a diminished ongoing pregnancy rate and a higher incidence of miscarriage.9 This is a clear limitation of vaginal progesterone designed for fresh transfer when used in the FET setting, remarked de Zeigler, and the core issue which must be solved in the modern freeze-all era.
Evidence from a recent study has shown the efficacy of SC progesterone in providing luteal-phase rescue in FET HRT cycles.17 In this study, progesterone was measured the day prior to embryo transfer and, if <8.75 ng/mL, was rescued with exogeneous progesterone provided in the form of daily SC injections at a dose of 25 mg. Following addition of SC progesterone, serum progesterone rose to 33.4 ng/mL on the day of embryo transfer and equivalent clinical pregnancy rates were achieved in the rescue group to the control arm: 55.0% versus 56.7%, respectively.17 Data from another study have also demonstrated the efficacy of SC progesterone given at double dose of two daily 25 mg injections in priming for FET. In this retrospective trial involving 214 women undergoing FET, priming with either SC progesterone (25 mg twice daily [BID]) or 90 mg vaginal gel (also given BID) resulted in equivalent reproductive outcomes. The live birth/ongoing pregnancy rate per embryo transfer was 39.3% with SC progesterone 50 mg BID versus 35.5% with vaginal supplementation.18
The only viable administration routes for progesterone in ART are parental and vaginal; oral and transdermic delivery are contraindicated due to poor bioavailability and permeability, respectively. IM injections were developed first for ART protocols but must be carried out by a nurse and are recognised to be painful by patients. The vaginal route was therefore introduced as an alternative. This results in a direct transfer of progesterone, leading to high local concentrations in the uterus, yet appears to underperform in FET, with around one-third of patients failing to reach sufficient levels of progesterone in the serum.
These problems encountered with vaginal progesterone have led the majority of groups in the USA to revert back to using exclusively IM progesterone for FET, or a combination of IM/vaginal regimens. However, de Ziegler explained that in the modern era of ART there is now access to a new injectable option for progesterone: an aqueous solution that has been developed specifically for SC delivery (Prolutex®; IBSA Institut Biochimique SA, Lugano, Switzerland). In this formulation, progesterone has been encapsulated in cyclodextrin, a polar substance long-used for enhancing the solubility of drugs, to overcome its inherent hydrophobicity. Once injected, the cyclodextrin envelope (a starch residue) is readily digested and progesterone released.
Pharmacokinetic analyses have compared SC progesterone 25 mg administered BID against both vaginal progesterone (90 mg) and IM progesterone (50 mg) given once daily (data on file).Currently, the SC progesterone preparation is approved at doses of 25 mg daily for fresh embryo transfers, but in FET “we need more,” reiterated de Ziegler. In this pharmacokinetic study, BID administration of SC progesterone achieved a higher rate of absorption compared to controls and delivered similar trough levels to IM injection (data on file).
Looking at clinical outcomes, SC progesterone (25 mg BID) was compared to IM progesterone (50 mg/day), the standard dose used at most ART centres, in a recent retrospective study involving >500 patients.19 Both formulations of progesterone were found to be equivalent in terms of pregnancy rate, live birth rate, and miscarriage rate.19 de Ziegler described this as a “major finding,” validating a new option for effective progesterone supplementation that avoids the painful IM injection and replaces it with a simple, self-administered SC injection, while simultaneously circumventing the well-recognised shortcomings of vaginal dosing.
A further prospective study, presented at the European Society of Reproduction and Embryology (ESHRE) Annual Meeting 2021, compared luteal support with IM progesterone 50 mg (n=92) to SC progesterone 25 mg BID (n=133) and also explored the association between progesterone blood levels achieved with each dosing route and outcome.20 Contrary to the clinical experience with vaginal dosing, there was no difference in ongoing pregnancy rate between the different progesterone level groups when ranked by quartiles in patients treated with SC progesterone.20 In all cases, the serum levels of progesterone reached with twice-daily SC injection were sufficient, explained de Ziegler, thereby negating the need for ongoing progesterone level monitoring in FET cycles. The same benefit was not seen for IM dosing, where progesterone levels on the day of transfer were still found to have a significant impact on pregnancy rate (p=0.02).20 This study also showed similar reproductive outcomes were achieved by patients treated with either daily IM or twice-daily SC progesterone injections.20 Clinical pregnancy rates were 64.7% versus 62.6% (p=0.757), miscarriage rates were 24.4% versus 17.5% (p=0.329), and ongoing pregnancy rates were 48.9% versus 51.6% (p=0.683), respectively.20
Although vaginal progesterone has been validated in fresh cycles as we move toward the freeze-all era and most of us now perform approximately 60% FETs, remarked de Ziegler, it must be acknowledged that vaginal progesterone has reached its limits. This poses an intellectual problem: why do low circulating blood levels of progesterone matter when we know high tissue concentrations are achieved with vaginal supplementation? de Ziegler suggested that the answer may lie in the pelvic and non-pelvic effects of progesterone. Progesterone not only acts on the uterus, but also works outside of the pelvis, where it exerts immunotolerance effects on non-pelvic organs, including the bone marrow and lymphocytes, adrenals, and the liver. This immunomodulation is a non-pelvic effect dependent on blood levels of progesterone and could therefore be the ‘Achilles heel’ of vaginal progesterone, de Ziegler proposed.
SC self-injected progesterone administered at a dose of 25 mg BID should therefore be considered the ‘true replacement’ for IM progesterone in FET cycles, de Ziegler concluded. SC injections provide a valid and efficient alternative to painful IM injections, which benefit from patient-friendly administration and have demonstrated comparable efficacy in priming for FETs. If using vaginal supplementation, an alternative solution is to measure progesterone and, if levels drop too low, rescue the cycle with SC progesterone, or use a combination regimen of vaginal plus one injection of SC progesterone daily in all patients, suggested de Zeigler, thereby eliminating the need to monitor progesterone at all.








