Meeting Summary
This article summarises a Theramex-sponsored symposium delivered on 9th May 2024 as part of the International Society of Gynecological Endocrinology (ISGE) Congress in Florence, Italy, between 8th–11th May 2024. A distinguished panel of experts elaborated on different aspects of cardiovascular health in women receiving menopausal hormone therapy (MHT). Rossella Nappi, Research Center for Reproductive Medicine and Gynecologic Endocrinology–Menopause Unit, IRCCS San Matteo Foundation, University of Pavia, Italy, chaired and opened the symposium with an overview of cardiovascular risk in women, particularly during the menopause transition, and described the benefit of oestrogen in mitigating cardiovascular risk. She was followed by Katrin Schaudig, Center for Gynecologic Endocrinology, Hormone Hamburg, Germany, who explained the importance of the choice of progestogen and the route of oestrogen administration in combined MHT in terms of risk of cardiovascular and other events. The final talk was given by Petra Stute, Gynecologic Endocrinology and Reproductive Medicine, Department of Obstetrics and Gynecology, University Clinic Inselspital Bern, Switzerland, who presented recent real-world data from the USA database to describe the risk of major cardiovascular events (MACE) in menopausal women treated with oral oestradiol/micronised progesterone in comparison to conjugated equine oestrogen (CEE) plus medroxyprogesterone acetate (MPA).
Introduction
Oestrogen deficiency during menopause is associated with a number of different symptoms, including vasomotor symptoms such as hot flashes and night sweats; psychological effects, such as sleep disturbances, anxiety, and mood changes; physical discomfort, such as joint and muscle pain; and genitourinary symptoms.1 It has also been shown that women with symptoms of menopause tend to have a lower health-related quality of life and an increased need for healthcare services compared with women without symptoms.1
Menopausal symptoms may be relieved by counteracting falling oestrogen levels through the use of MHT.1 However, despite the evidence supporting the use of MHT, uptake of this therapy remains low, with many women expressing ongoing concerns about adopting MHT.1,2 This is largely a legacy of the 2002 Women’s Health Initiative (WHI) study of CEE/MPA in post-menopausal women, which was terminated prematurely due to an increased risk of breast cancer with no improvement in cardiovascular risk.3 In the years since the early discontinuation of the WHI study, progress has been made in understanding the risk–benefit profile of MHT in terms of its timing and duration of use, and also in how body-identical MHT may offer benefits over conventional non-body-identical MHT.1 Several international societies recognise MHT as an effective option for alleviating menopausal symptoms by addressing declining oestrogen levels.1,4-7 Women with an intact uterus are recommended to receive MHT in the form of oestrogen combined with a progestogen to protect the uterus from endometrial cancer, while women who have had a hysterectomy are prescribed oestrogen alone.1
This symposium explored the importance of tailoring MHT to the needs of individual women, including the impact of type of progesterone on cardiovascular risk.
Menopause and Cardiovascular Disease
Nappi presented 2020 US mortality data to show that, although heart disease and stroke currently claim more lives each year than cancer and chronic lower respiratory disease combined,8 there was a reduction in the percentage of US women identifying heart disease/heart attack as the leading cause of death between 2009–2019, but an increase in those identifying cancer and breast cancer as leading causes.9
Declining oestrogen levels and increased abdominal fat in postmenopausal women lead to unfavourable metabolic changes, resulting in increased cardiovascular risk.10 In particular, there is a significant acceleration in cardiovascular risk over the course of the menopause transition when levels of oestrogen are declining, with the rate of events increasing beyond what would be expected for chronological ageing alone.11 During this phase of a woman’s life, cardiometabolic changes can be separated into those associated with chronological ageing and those that are due to ovarian ageing, or a combination of the two.12
Certain categories of women, including those with elevated BMI, increased waist circumference, unfavourable metabolic profile, hypertension, and unchanged cycle length over the menopausal transition, have a higher risk of cardiovascular disease.10 In addition, the Framingham study showed that those entering menopause before the age of 40 years had a four-times increased risk of cardiovascular disease,13 and a pooled analysis of 301,438 women showed that women with menopause before the age of 40 years had a significantly higher risk of cardiovascular disease compared with those who had menopause at age 50–51 years (hazard ratio [HR]: 1.55; 95% CI: 1.38–1.73; p<0·0001).14 Women with obesity also have a 64% increased risk of coronary heart disease compared with 46% among men who are obese, and female smokers have a 25% greater risk of cardiovascular disease.15
These findings highlight the importance of ascertaining a full medical history to assess overall lifelong cardiovascular health in order to identify women with higher risks at menopause.16,17 The Lancet Women and Cardiovascular Disease Commission issued recommendations to reduce the global burden of cardiovascular disease across the entire lifespan of women by 2030, focusing on sex-specific differences in cardiovascular risk factors.18,19
Menopausal Hormone Therapy Benefits and Risks: The Choice of Progestogens Matters
Schaudig began her presentation by noting that MHT may offer both short-term benefits, including relief of menopause symptoms, as well as possible long-term benefits, including protection against osteoporosis-related fractures.4 She highlighted that, for women with an intact uterus, MHT needs to be a combination of oestrogen and progestogen to protect against endometrial cancer.3,20,21
Schaudig noted that the route of administration of oestrogen has an impact on risk of adverse outcomes with MHT. Four separate studies reported a lower risk of venous thromboembolism (VTE) with transdermal oestradiol compared with oral oestradiol,22-25 while a case-control study showed an increased risk of ischaemic stroke with oral but not with transdermal oestrogens.26 Furthermore, the 2020 recommendations from the British Menopause Society (BMS) and Women’s Health Concern (WHC) on hormone replacement therapy in menopausal women explicitly recommend the use of transdermal oestradiol to mitigate the risk of VTE and stroke in women with related risk factors.27
As well as the route of administration of oestrogen, the type of progestogen also appears to affect the risk of adverse outcomes. A large number of different types of progestogen are available, each with a distinct biological and clinical profile depending on its tissue concentration and receptor-binding affinity.28 The WHI evaluated the effects of CEE plus MPA versus placebo among 16,608 post-menopausal women aged between 50–79 years in the USA.3 The study showed that absolute excess risks per 10,000 women-years attributable to CEE plus MPA were seven more coronary heart disease events, eight more strokes, eight more pulmonary embolisms (PE), and eight more invasive breast cancers, with decreases in the number of events per 10,000 women-years in colorectal cancers (six fewer) and hip fractures (five fewer), compared with placebo.3 A subsequent subgroup analysis showed that the risk–benefit profile of CEE alone was favourable for all outcomes except VTE and stroke, including a decreased number of breast cancer events.29,30
As described earlier, the WHI also showed an increased risk of deep vein thrombosis and PE with CEE plus MPA versus placebo,3 while the addition of micronised progesterone to transdermal oestradiol does not appear to increase the risk of VTE.22 The case-control study described earlier showed an increased risk of ischaemic stroke with norpregnane derivatives in combined MHT, but not with progesterone, pregnane derivatives, or nortestosterone derivatives.26 Finally, evidence from the French case-control study ESTHER (Estrogen and Thromboembolism Risk) and the French E3N cohort study showed that, unlike progesterone and pregnane derivatives, use of norpregnane derivatives in oral MHT increased risk of venous thromboembolism in post-menopausal women.31
Type of progestogen also appears to have an impact on breast cancer risk. The E3N Study reported an HR for development of invasive breast cancer following at least 5 years of treatment with oestrogen plus synthetic progestogen of 2.02 (95% CI: 1.81–2.26) compared with 1.31 (95% CI: 1.15–1.48) for micronised progesterone, which is chemically and biologically identical to endogenous progesterone.32 This suggests that using micronised progesterone with oestradiol may have a different risk profile for breast cancer compared to synthetic progestogens.32 The difference in breast cancer risk between MPA and MP in combination with oestrogen has further been confirmed in meta-analyses up to 5 years33 and a randomised controlled trial with a median follow-up of 5 years.34
An additional potential benefit of oral micronised progesterone is promoting quality sleep in menopausal women. In randomised controlled trials predominantly enrolling post-menopausal women, oral administration of micronised progesterone had a measurable benefit on various sleep outcomes, possibly as a result of its gamma-aminobutyric acid type A (GABA) receptor-modulation activity.35
Because the route of oestrogen administration and choice of progestogen both have such a profound impact on patient outcomes, it is essential to individualise therapy such that it is tailored to patient risk factors, comorbidities, and family history. As progestogens are essential in MHT for menopausal women with a uterus to prevent endometrial hyperplasia and reduce cancer risks, it is important to select a progestogen with a favourable safety profile.22 Micronised progesterone appears to be an optimal choice for women in special situations, such as in the presence of cardiovascular disease, high-density breast tissue, obesity, and risk of VTE.36 Furthermore, the use of a transdermal oestrogen should be considered in women with related risk factors, such as a history of VTE, uncontrolled diabetes, hypertension, or obesity.36
Real-world Evidence: Incidence of Cardiovascular Major Adverse Events of Oestradiol/Micronised Progesterone in Comparison with Conjugated Equine Oestrogen/Medroxyprogesterone Acetate
Stute reminded the audience that the WHI showed an increased risk of cardiovascular diseases and VTE with CEE/MPA compared with placebo.3 However, she noted that we now have a much greater understanding of the importance of the choice of progestogen on cardiovascular risk, and observed that micronised progesterone has a different safety profile compared to synthetic progestins such as MPA.37 The Phase III randomised double-blind placebo-controlled study investigating a single oral capsule of oestradiol/micronised progesterone combined MHT (E2/P4) showed no clinically significant changes in coagulation or metabolic parameters for E2/P4 compared with placebo over 12 months of observation.38,39 A 2023 study of 36,061 women, in which the analyses were weighted by the inverse probability of treatment for control of potential confounding factors, showed a significantly lower incidence of VTE for oral E2/P4 compared with oral CEE/MPA.40 However, data comparing E2/P4 with CEE/MPA on the risk of MACE incidence are currently lacking. Therefore, the first head-to-head retrospective, longitudinal study of MACE incidence in menopausal women treated with E2/P4 versus CEE/MPA was undertaken, with the results reported for the first time at the symposium.41
The study was a retrospective observational investigation assessing claims from a US database capturing data between April 2019–June 2021. This large study included around 36,000 women treated with 17β-oestradiol E2/micronised progesterone P4 (E2/P4) (combined body-identical MHT) or CEE/MPA in a real-world setting, and followed the same design as the previous study investigating the rate of VTE with E2/P4 compared with CEE/MPA (Figure 1) (Stevenson et al., Unpublished data).40,41
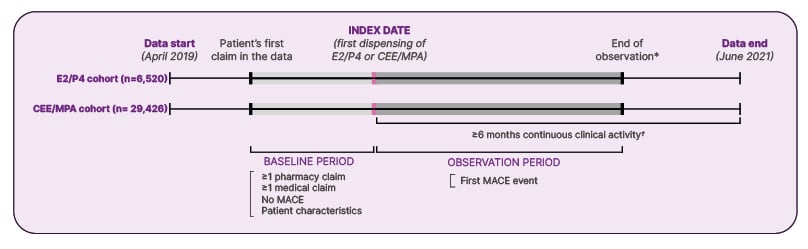
Figure 1: Study design: retrospective observational study of US claims database (major adverse cardiovascular events study) (Stevenson et al., Unpublished data).
*Earliest of index treatment from E2/P4 to CEE/MPA, or from CEE/MPA to E2/P4, data cut-off date, or end of clinical activity.†
†Pharmacy-based activity was defined as no gap ≥12 months between two prescription claims (for hormone therapy or other drugs); medical-based activity was defined as no gap ≥12 months between two medical claims.
CEE: conjugated equine oestrogen; E2: oestradiol; MACE: major adverse cardiovascular events; MPA: medroxyprogesterone acetate; P4: micronised progesterone.
Women were eligible for the study if they were aged at least 40 years, with at least one prescription for E2/P4 or CEE/MPA. Women were required to have at least one medical claim and at least one pharmacy claim before the index date, as well as no hospitalisation with a MACE diagnosis (acute myocardial infarction [ICD-10 diagnosis codes: I21.x, I22.x, or procedure code for revascularisation procedure]; ischaemic or haemorrhagic stroke [ICD-10 diagnosis codes: I61.x, I62.x, I63.x, I64.x]; or heart failure [ICD-10 diagnosis codes: I50.x, excluding I50.x2 and I50.8x]). Women were excluded if they had had a MACE event in the baseline period or before the index date, or had switched from E2/P4 to CEE/MPA or from CEE/MPA to E2/P4 in the 6 months following the index date (Stevenson et al., Unpublished data).
An inverse probability of treatment weighting (IPTW) analysis of the baseline characteristics of the MACE study revealed that the mean age of the women was 54.7–55.8 years, and a high proportion of participants had cardiovascular disease (39.7–41.9%), diabetes (10.7–11.1%), or hypercholesterolaemia (28.1–29.4%) at baseline (Stevenson et al., Unpublished data). Approximately 60% of women in both groups were taking treatment for sleep disorders, depression, or anxiety, and around 40% were taking analgesics or relaxants in the post-IPTW analysis (Stevenson et al., Unpublished data).
The results indicated that women treated with E2/P4 had a significantly lower risk of MACE events by 72% compared with CEE/MPA (p<0.05; Figure 2) (Stevenson et al., Unpublished data). Analysis of the number of MACE events per 10,000 women/year showed an early divergence of the two rates curves.
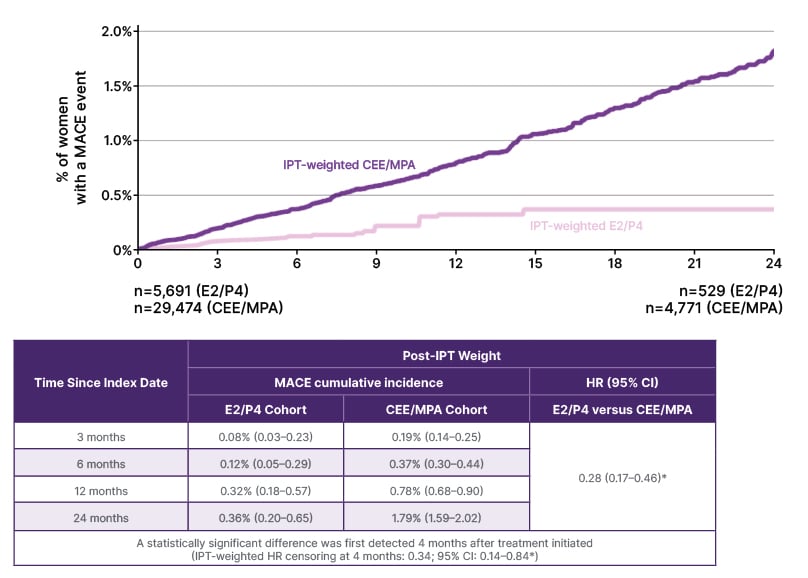
Figure 2: Major adverse cardiovascular events rates following inverse probability of treatment for oestrogen/micronised progesterone versus conjugated equine oestrogen/medroxyprogesterone acetate (weighted analysis) (Stevenson et al., Unpublished data).
*Statistically significant at p<0.05.
CEE: conjugated equine oestrogen; E2: oestradiol; HR: hazard ratio (E2/P4 versus conjugated equine oestrogen/medroxyprogesterone acetate); IPT: inverse probability treatment; IRR: incidence rate ratio (E2/P4 versus CEE/MPA); KM: Kaplan-Meier; MPA: medroxyprogesterone acetate; P4: micronised progesterone; WY: women-years.
The effect was consistent across individual MACE outcomes, with significantly more events in the CEE/MPA group than the E2/P4 group for heart failure, acute myocardial infarction, and stroke among women aged over 40 years (Figure 3) (Stevenson et al., Unpublished data). The effect was also consistent across all age subgroups, although a greater difference in the rate of MACE between the two treatment groups was apparent among older women, with IPTW analyses stratified by age showing higher MACE event rates in women aged 60–79 years than those aged 40–59 years (MACE events per 10,000 women-years: 39.5 versus 23.9 for E2/P4 and 145.8 versus 61.6 for CEE/MPA, respectively) (Stevenson et al., Unpublished data).
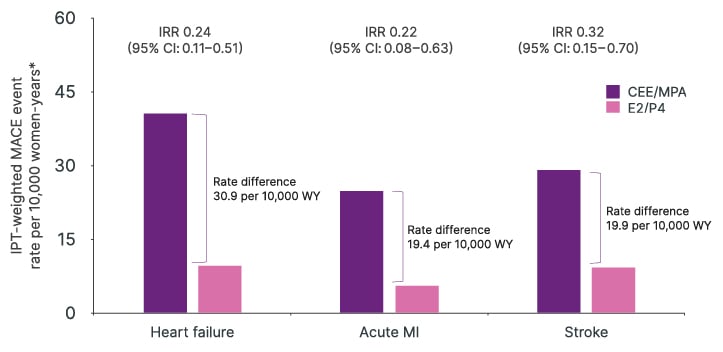
Figure 3: Major adverse cardiovascular events rates following inverse probability of treatment for oestrogen/micronised progesterone versus conjugated equine oestrogen/medroxyprogesterone acetate by individual event (weighted analysis) (Stevenson et al., Unpublished data).
*MACE event indexed to person-time post-index date; first MACE event included in the rate numerator; women-time up to first event or until end of observation was included in the denominator.
CEE: conjugated equine oestrogen; E2: oestradiol; IPT: inverse probability treatment; IRR: incidence rate ratio (E2/P4 versus CEE/MPA) (estimated from IPTW Poisson/negative binomial regression models); MACE: major adverse cardiovascular events; MPA: medroxyprogesterone acetate; P4: micronised progesterone; WY: women-years.
In terms of the earlier VTE study, baseline characteristics revealed that despite the relatively young age of patients in both cohorts (mean age: 54.9–55.9 years), there was a high incidence of cardiovascular disease (40.9–42.1%), diabetes (11.2–11.3%), hypercholesterolaemia (28.7–29.6%), and obesity (9.8–11.0%).40 A post-IPTW analysis revealed that VTE rates were statistically lower for E2/P4 compared with CEE/MPA (IPTW HR: 0.70; 95% CI: 0.53–0.92).40
It should be noted that there are a number of limitations associated with the real-world studies, including the fact that administrative claim databases may contain errors or omissions in codes for diagnoses, dispensation, or procedures (Stevenson et al., Unpublished data).40,42-44 Furthermore, MACE events may have been underestimated because the date of death is not captured in the data, while the rate of VTE events may be underestimated as only the first VTE event after the index date was counted in the analyses due to difficulties in separating new VTE events from subsequent visits for VTE follow-up in the claims data (Stevenson et al., Unpublished data). Finally, residual confounding may have occurred from MACE and VTE risk factors not available in claims data (Stevenson et al., Unpublished data).40
Stute concluded that the new real-world data on MACE rates combined with earlier VTE data demonstrate a statistically significant reduction in the rate of MACE events and VTE events associated with E2/P4 compared with CEE/MPA in clinical practice (Stevenson et al., Unpublished data).40 Although further studies are needed to explore this hypothesis, these results highlight the importance of choosing an optimum progestogen with a favourable safety profile as part of combined MHT, and in tailoring treatment to ensure the best outcomes for women.
| Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow card in the Google Play or Apple App store.
Adverse events should also be reported to Theramex on [email protected] or Tel: +44 (0)333 0096795 |







