The human body is colonised by many more bacteria than there are human cells. In addition to bacteria, there are other micro-organisms, although less abundant, such as viruses, fungi, microscopic eukaryotes, and archaea that contribute to the general microbiota. Recent research has highlighted the role of the microbiota in regulating human physiology in health and disease.1,2
Considering the abundance of microbiota, the totality of micro-organisms and their collective genetic material present in the human body, known as the human microbiome, has even been termed the second genome. The body’s second genome offers new insights into the physiological condition and the dynamics between homeostasis and the pathogenesis of disease, with further extension to the promise of novel diagnostic and therapeutic approaches.
The female reproductive tract, specifically the vaginal milieu, has long been known to have an active microbiota. Lactobacilli are the cornerstone species in women of reproductive age, with studies in the USA determining that 3–4 community types of vaginal microbiota, out of 5 types identified, contain >90% Lactobacillus.3 The lactic acid produced by the vaginal microbiota helps to maintain a low pH of 3.5–4.5, a major factor in limiting the growth of potentially harmful bacteria. Alterations in the vaginal microbiota play a role in common conditions, such as bacterial vaginosis, sexually transmitted diseases, urinary infections, and preterm birth.4-7
The uterus, on the other hand, was considered to be sterile until recently. The sterile womb paradigm, coined by Henry Tissier in 1900, was a commonly believed dogma that theorised that human infants develop within a sterile environment.8 However, the assumption of a sterile, healthy uterus was challenged by multiple reports in the second part of the 20th century, which used culture-dependent methods to show bacterial colonisation varied from 0–90%.9 With the advent of next-generation sequencing technologies in 2007, the belief that a healthy uterus is sterile has been revisited by recent studies, and it is now becoming clear that the uterus has micro-organisms with roles beyond infection. It is now acknowledged that only ~1% of bacteria are culturable,10 and sequencing the unique bacterial 16S ribosomal RNA gene has resulted in an explosion in research to define microbial communities. Nevertheless, when applying next-generation sequencing we should be aware that the occurrence of a sole bacterial DNA is not consistent with the presence of living forms of the micro-organisms. In other words, sequencing results do not enable us to distinguish between alive or dead bacteria within the sample, which is important information from a microbiological, clinical, and ecological point of view.
Recent studies have identified unique uterine microbiota that differ from that of the vagina;9 however, the estimations of uterine bacterial load are estimated to be 100–10,000-times lower than that of the vaginal microbiome.3,11 A pioneering study in the field analysed the microbiota along the female reproductive tract in 95 women of reproductive age.3 The results showed that, contrary to the vaginal and cervix microbiota, Lactobacillus do not dominate and bacteria such as Pseudomonas, Acinetobacter, Vagococcus, and Sphinogobium constitute a notable fraction of the uterine microbiome (Figure 1). These bacteria grow in mildly alkaline conditions, contrasting to the Lactobacillus-dominated low pH environment of the vagina.
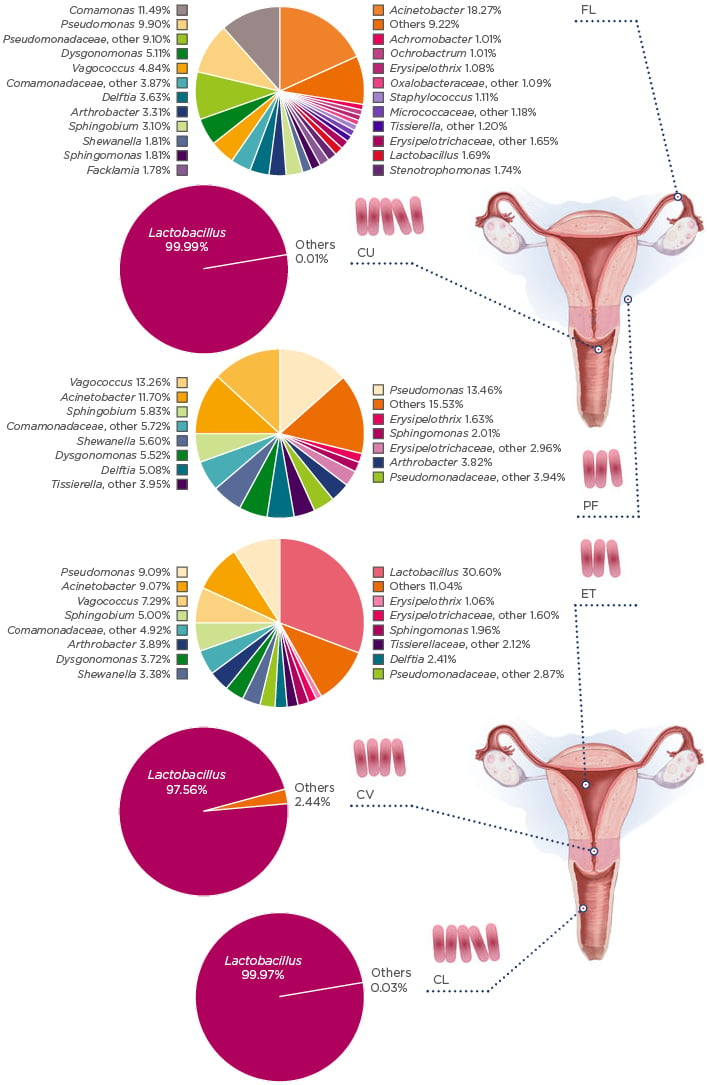
Figure 1: The microbiota continuum along the female reproductive tract.
CL: lower third of vagina; CU: posterior fornix; CV: cervical mucus drawn from cervix; ET: endometrium/uterus; FL: fallopian tubes; PF: peritoneal fluid from the Pouch of Douglas.
Adapted with permission from Chen et al.10 The figure is reproduced with permission from Nature Publishing Group.
WHERE DO THEY COME FROM?
The microbiota in the uterus can originate from different sources and migrate to the uterus via various methods, including haematogenous spread, ascension from the vagina, via the sperm, and other methods (e.g., retrograde spread through the fallopian tubes or gynaecological procedures) (Figure 2).9,12 Colonisation of specific bacteria via the haematogenous route has been shown in mice.13 Bacterial spread through the bloodstream via either an oral route14 or the gut15 enables bacteria from mucosal sites, such as the oral cavity and gastrointestinal tract, to colonise distal mucosal sites, and this occurs during epithelial barrier breach.13 Bacterial viability has been shown to be conserved during translocation through the blood, with intracellular dormancy being one way bacteria remain viable in the blood.9
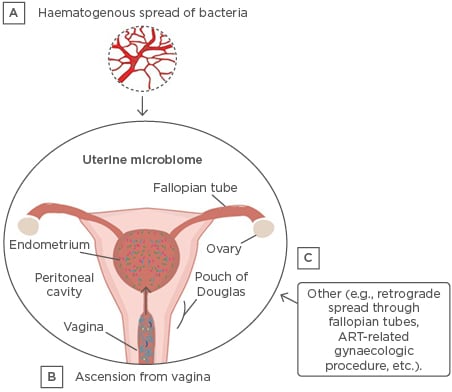
Figure 2: Established and putative bacterial transmission routes between uterine microbiota and distal sites.
ART: assisted reproductive technologies.
Adapted from Baker et al.10 The figure is reproduced with permission from Nature Publishing Group.
Ascension of bacteria via the cervix has been well-established and is another highly probable source of bacterial transmission.9 Other sources of uterine microbiota seeding may originate from assisted reproductive techniques, whereby bacteria from the vagina are introduced into the uterus (e.g., oocyte retrieval and embryo transfer) or during the placement of intrauterine contraceptive devices.16,17 Additionally, there is a factor of sexual intercourse that can influence the uterine microbiota; it has been shown that sexual intercourse influences the vaginal microbiome7,18 and, more specifically, that semen and vaginal microbiomes are in association.19
MICROBIOTA MAY MODULATE IMMUNITY IN THE UTERUS
Although the exact role and mechanisms of micro-organisms in the uterus are unknown, new studies have suggested that microbiota could be responsible for a receptive, fertile endometrium by influencing uterine immunity.20 The immune system is involved in all aspects of reproductive success, especially during the time of conception and in the peri-implantation period,21 and it has been shown that local and systemic immunity is greatly influenced by microbiota.22 Indeed, lessons learned from the gut microbiome suggest that the microbiota of the uterus could modulate immune cells involved in implantation and have implications for tissue morphology.20 Microbiota can also be important in protection against infections by competing with invading pathogens in the uterus.20
UTERINE MICROBIOTA IN HEALTH AND DISEASE
To date, the main focus of uterine microbiota studies has been on the negative consequences of the presence of bacteria, and fewer studies have assessed the microbiome of a healthy uterus. The most abundant bacteria detected in the uterus belong to the phyla Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria.9 However, the few studies analysing uterine microbiota in healthy asymptomatic women using next-generation sequencing show little consistency,9 and further studies with larger sample sizes and including different ethnicities are needed to establish the normal or core uterine microbiota.
The presence of bacteria in the uterus has been associated with different gynaecological complications, including poor reproductive outcomes,23-26 endometriosis,10,24 dysfunctional menstrual bleeding,27 and cancer;28 nevertheless, a cause and effect relationship has not been clearly established. Uterine colonisation with bacterial vaginosis-associated bacteria has been suggested to promote carcinogenesis through microbiota-mediated pathophysiologic changes.21,22 For example, a recent study28 analysed the microbiome at various sites in the female reproductive tract and associated the presence of Atopobium vaginae and Prophyromonas species in the reproductive tract with cancer.
In women with endometriosis, the uterine microbiota composition has been shown to be different compared to healthy controls;10,23 in these women, Lactobacillaceae are present in lower levels and Streptococcaceae, Staphylococcaceae, and Enterobacteriaceae species are enriched.29
A reduction in clinical pregnancy rates has been reported when bacteria were cultured from the in vitro fertilisation (IVF) catheter tip during assisted reproductive technology procedures,30 which can seed the uterine microbiota and cause adverse reproductive and gynaecological outcomes by modulating the local microenvironment.16 Other studies have detected a unique endometrial microbiota dominated by Bacteroides residing in the endometrium of women with different reproductive failures,26 or no differences in endometrial microbiota at the moment of embryo transfer between infertile women with ongoing pregnancies and those without pregnancy.23 In these women, Firmicutes (Lactobacillus) and Bacteroidetes (Flavobacterium) phyla represented the majority of the bacteria. The fact that Bacteroides regulate certain mechanisms in the gut that are relevant to the endometrium is intriguing, including mechanisms such as mucosal barrier reinforcement, epithelial cell maturation, and maintenance and interactions with the host immune system to control other bacteria.31 Nevertheless, there is only one study25 to date that has detected a statistical difference in microbiome profiles between successful and unsuccessful reproductive outcomes, rather than the mere presence of bacteria in the uterus. The study analysed 35 infertile women undergoing IVF (the biggest sample size analysed in this type of study) and the results demonstrated that non-Lactobacillus-dominated microbiota were associated with decreased implantation, pregnancy, and live birth rates among infertile women undergoing IVF.25
Larger sample sizes and standardised procedures to avoid or minimise vaginal cross-contamination are important aspects for future studies in determining the role of the uterine microbiota in reproductive health and disease.
CONCLUSION
The first studies of the uterine microbiota have highlighted significant changes in bacterial community compositions related to different gynaecological diseases, rates of IVF success, and risk of endometrial cancer. These pioneering studies provide a starting point for future research into the uterine microbiota to understand uterine physiology in health and disease, provide potentially effective clinical interventions for a variety of conditions, and have a positive impact on obstetric and gynaecological health.
In addition to the bacterial communities, other micro-organisms such as viruses, fungi, archaea, and microscopic eukaryotes exist in the uterine microflora that need to be investigated. Future studies should also clarify if the micro-organisms present in the uterus are residents that maintain homeostasis, tourists that are easily eliminated, or invaders that contribute to reproductive diseases. Furthermore, there is a need for studies investigating host–microbiota interactions and the physiologic and functional impact of the micro-organisms on the local endometrial microenvironment because these mechanisms may influence poor reproductive, obstetric, and gynaecological health outcomes.
As with any new research area, there are endless questions to be answered, but the uterine microbiota seems to have a high potential for providing additional knowledge of female reproductive functions and to predict and to improve its outcomes. It is time to consider micro-organisms not only as enemies but also as allies in reproductive medicine.








