INTRODUCTION
The field of reproductive medicine is recognised for its rapid innovations and advanced technologies, but also for its connection with lucrative medical industry and marketing. In vitro fertilisation (IVF) patients deal with multiple challenges when facing fertility treatment which have a limited success and come at a huge cost. In this context, IVF centres offer, and patients ask for, a spectrum of unproven add-on interventions, called ‘add-ons’, to increase the chances of a having a live birth.1
The Human Fertilisation and Embryology Authority (HFEA) recently produced a statement analysing 12 add-ons to disseminate clear patient information.2 For this purpose, patient-friendly communication strategies have been adopted using a traffic-light rating system that represents how effective add-ons are. Thus, based on the level of evidence, the following system has been used: i) colour green when more than one good-quality randomised controlled trial (RCT) is present in the literature, indicating that the procedure is effective and safe; ii) colour amber when further studies are required due to limited body of evidence, showing that the procedure cannot be considered as standard therapy; and iii) colour red when no evidence of safety and efficacy exists. Although the HFEA tried to guide both professionals and patients on the principles that should be followed when choosing whether or not to use an add-on therapy with IVF treatment, the HFEA statement did not consider aspects other than efficacy and did not discriminate between short-term and long-term data regarding children’s safety.
For this aim, the authors used a model framework proposed by the European Society of Human Reproduction and Embryology (ESHRE) in 2014 to distinguish when specific add-ons fall in the ‘experimental treatment’ category and when they can be moved into the ‘innovative treatment’ or ‘established treatment’ categories.3 This tool, proposed by the Special Interest Group ‘Safety and Quality in Assisted Reproductive Technology (ART)’ and ‘Ethics and Law’ and described by Provoost et al.,3 is based on a scoring system assessing four criteria: efficacy, safety, procedural reliability, and effectiveness. The choice for using this tool to categorise the IVF add-ons and compare the classification with the traffic light system used by the HFEA is based on the fact that the Provoost model is a consensus-based model. A group of experts has prepared the framework based on the problematic dichotomy between experimental and established technologies. This dichotomy does not include the innovative treatments in clinical practice. The tool gives clarity on the notion of experimental and innovative treatments without deciding to restrict these treatments. The latter is not the purpose of the model proposed by Provoost et al.3 Moreover, this paper gives guidance on how to implement the classified treatments and to strictly follow-up patients. The classification of the treatments is based on four criteria; the efficacy of the procedure (criterion 1) is scored with 0 when no proof of principle has been demonstrated or with 1 when it is.3 While the efficacy is a categorical criterion (pass or fail), the other three criteria are ordinal. Safety (criterion 2) is referred to patients as well as the future children, considering safe data in animals (score 1), reassuring preclinical, short, mid, and long-term data (scores 2, 3, 4, and 5, respectively).3 The different degrees of procedure variability (criterion 3) in different laboratories are taken into account by the procedural reliability, characterised by procedures enormously or highly variable between laboratories (score 1–2) to procedures considered as routine techniques (score 5).3 The effectiveness (criterion 4) is referred to as the likelihood of producing the desired outcome compared with outcome of established ART techniques (from unknown/low likelihood with score 1–2 to high or higher than conventional ART techniques with score 5).3 To define a treatment from experimental through innovative to an established one, it is important to assess these four criteria considering evidence-based medicine literature.3 For each of the criteria a threshold exists, and if a treatment scores below the threshold, even for just one of the criteria, it cannot be moved to the subsequent definition, even though it would score higher for other criteria (Figure 1).3 Only treatments scored above the thresholds for all the four criteria could be considered innovative treatments (efficacy score 1, safety score 3, procedure reliability score 2, and effectiveness score 2). When treatments showed a score of ≥4 on the last three criteria, they could be considered established treatments (Figure 1).3
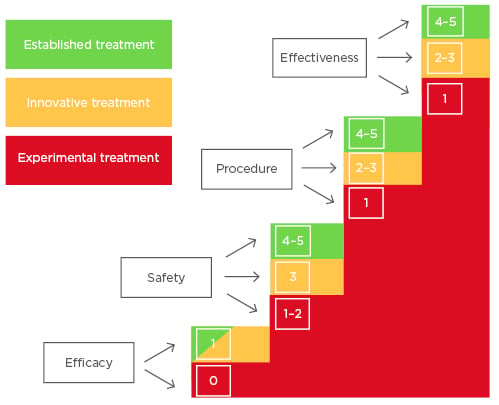
Figure 1: Sequential four-criterion assessment tool to consider the transition of a treatment from experimental (colour red) through innovative (colour amber) to established one (colour green) with the related scores.
This paper describes the application of the four-criterion assessment tool to the 12 add-ons reported by the HFEA, analysing the add-ons with the Provoost model and comparing them to the traffic light representation of the HFEA.
ASSITSTED HATCHING
The artificial rupture of the zona pellucida to improve the chances of implantation and clinical pregnancy is known as assisted hatching (AH) and it is characterised by multiple different manipulations, i.e., making holes, thinning, or breaching of different sizes, or using laser, mechanical, or chemical methods on fresh or frozen/thawed embryos at different developmental stages.4 Although a significant number of RCT is available, these studies are heterogeneous and do not support the efficacy of the technique. As a matter of fact, some meta-analyses were performed to evaluate the effect of embryo AH on clinical outcomes, reporting a borderline significant improvement in clinical pregnancy while the live birth rate has still not proven to be increased by AH.5,6 Although very few RCT reported an absence of embryo damage and baby’s congenital anomalies, a retrospective cohort study with a large sample size also suggested that AH alone does not increase the risk of major congenital anomalies when looking at 36,033 births after AH.5,7
The National Institute for Clinical Excellence (NICE) guidelines do not recommend the use of AH to improve pregnancy rates.8
Since only neonatal outcomes related to AH have been reported, the AH procedure should be considered as an experimental treatment based on the Provoost model. To move from experimental to innovative treatment on the continuum, the safety threshold should be characterised by reassuring short-term data on children up to at least 3 months post-delivery (score 3).3
Likewise, the HFEA states that there is no evidence available supporting the effectiveness of this add-on, and hence defined it as a red-coloured traffic light (Table 1).
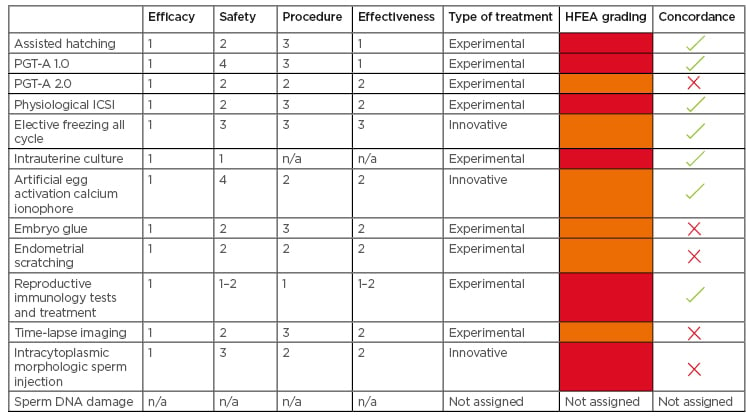
Table 1: Evaluation of 12 add-ons using European Society of Human Reproduction and Embryology (ESHRE) sequential four-criterion assessment versus Human Fertilisation and Embryology Authority (HFEA) grading.
HFEA: Human Fertilisation and Embryology Authority; ICSI: intracytoplasmic sperm injection; PGT: preimplantation genetic testing; n/a: not applicable.
PREIMPLANTATION GENETIC TESTING FOR ANEUPLOIDIES
To identify aneuploid embryos that are unsuitable for embryo transfer, 11 RCT based on Day 3 embryos analysed by fluorescence in situ hybridisation (FISH) showed no increase in live birth rate and, in some cases, a decrease in outcome.9 As such, this first version of preimplantation genetic testing for aneuploidies (PGT-A 1.0) using FISH has been neglected. Nowadays, the PGT-A implies the comprehensive chromosome screening of biopsied trophectoderm cells of blastocysts (PGT-A 2.0). Regardless of these improvements, the real clinical value of PGT-A 2.0 is still controversial. In fact, PGT-A 2.0 has increased the implantation rate, reduced the miscarriage rate, and decreased the time to have a baby.10 Unfortunately, the level of evidence seems to be low, attributable to the paucity of RCT (three RCT in good prognosis patients and one RCT in advanced maternal age women).10 Moreover, no studies regarding the long-term follow-up of children born after PGT-A 2.0 at the blastocyst stage are currently available.10 While PGT-A 1.0 was categorised red by HFEA and could be considered an experimental treatment because of lack of evidence on its effectiveness (score 1), the PGT-A 2.0 has been assigned the amber colour by the HFEA. PGT 2.0 could be defined as an experimental treatment in the Provoost model. In fact, there are reports showing some effectiveness (score 2), reassuring preclinical data on children (score 2), and that there is still variability in the procedures (score 2).10 (Table 1).
PHYSIOLOGICAL INTRACYTOPLASMIC SPERM INJECTION
Hyaluronan-based selection of sperm, so-called physiological intracytoplasmic sperm injection (PICSI), is a strategy to select the best sperm for ICSI through the binding between hyaluronic acid and the sperm plasma membrane. The Cochrane review based on two RCT concluded that evidence was insufficient to show any difference in clinical pregnancy and live birth rates between PICSI and standard ICSI and no clear data on adverse effects are available.11 Recently, a robust multicentre RCT demonstrated that PICSI did not significantly increase the live birth rate compared to ICSI.12 Although this study was not powered to investigate miscarriage, a significant reduction in miscarriage rate in the PICSI group compared with ICSI group had been observed. Nevertheless, the authors concluded that the wider use of advanced sperm selection techniques for assisted reproduction was not recommended.12 The level of evidence is hence too low to demonstrate that this strategy is effective. Thus, HFEA associated the red colour to this strategy. Although PICSI represents a comparable procedure among laboratories (score 3), there is no short-term follow-up data (score 2), the effectiveness is low (score 2), and thereby could be considered an experimental approach when the Provoost model is applied (Table 1).
ELECTIVE FREEZE ALL CYCLE
With advances in cryopreservation of human embryos, the number of frozen–thawed embryo transfer (FET) cycles has increased steadily, with success rates after FET similar to those of fresh embryo transfer. This led to the development of the so-called ‘freeze-all strategies’ in IVF, in which the entire cohort of embryos is electively cryopreserved, and the transfer is delayed. This approach is preferred in patients at risk of developing ovarian hyperstimulation syndrome13 or undergoing PGT cycles. Recently, the elective freeze-all strategy has become more common and it is often offered to patients in order to perform the transfer of a cryopreserved embryo into a more physiologic environment, without the effect of ovarian stimulation on endometrial receptivity. The Cochrane review based on four RCT concluded that elective FET strategy (eFET) is not superior to fresh transfer in terms of cumulative live birth rates with a moderate-quality evidence, but it is associated with lower rates of ovarian hyperstimulation syndrome and a higher rate of pregnancy complications.14 Moreover, other RCT have been performed but with conflicting results. Recently, a robust RCT showed that frozen single blastocyst transfer resulted in a higher singleton live birth rate than fresh single blastocyst transfer did in ovulatory women with good prognosis.15 Furthermore, some studies have shown that frozen single blastocyst transfer led to a higher singleton birthweight, which was accompanied by a higher risk of pre-eclampsia.15 A register-based study on 4,758 children showed that health indicators were similar among FET and fresh ET singletons during a 3-year follow-up.16 Scoring safety, procedure, and effectiveness with score 3, it is possible to consider the eFET as an innovative treatment, and similarly HFEA assigned the amber colour (Table 1).
INTRAUTERINE CULTURE
There has been an attempt at recreating the in vivo embryo development conditions and transpose these to the in vitro embryo culture. The in utero encapsulation technology introduces microinjected human oocytes into a retrievable and permeable tubing system that is inserted in the uterus. This allows the optimal exchange between the uterine maternal environment and the developing embryo. Because no RCT exist, HFEA considered this strategy as red. Moreover, very little evidence regarding safety and no reassuring preclinical data are available using the device.17 Currently, the intrauterine culture could be considered as an experimental treatment (Table 1).
ARTIFICIAL EGG ACTIVATION CALCIUM IONOPHORE
Calcium ionophores are used as artificial oocyte activators to improve fertilisation rate (e.g., in a cycle subsequent to a total fertilisation failure or lower fertilisation rate). Two meta-analyses have been published.18,19 In the latest meta-analysis, supported by the meta-regression analysis, artificial oocyte activation (AOA) appeared to improve the overall pregnancy rate and live-birth rates after ET.18 Nevertheless, it is important to note that in the same study, no evidence supporting the use of AOA in IVF treatments was observed in a subgroup analysis based on only four RCT. Reassuring mid-term data have been reported in children, aged 3–10 years, born after AOA (score 4 for safety). The neonatal, neurodevelopmental, and behavioural outcomes were within expected ranges.20 The insufficient evidence to support the overall use of AOA is related to the heterogeneity in the AOA protocols and the indications (score 2 for procedure reliability and score 2 for the effectiveness). HFEA assigned the amber colour to the AOA treatment due to scanty data. Basing on the four-criterion assessment tool it can be considered an innovative treatment (Table 1).
EMBRYO GLUE
The use of hyaluronan-rich (HA) supplemented medium is proposed to increase the chance of the embryo adhering to the endometrium and is stated to improve live birth rate. Benefits in terms of outcomes using HA-enriched transfer media still remain controversial.1 The latest Cochrane review reported the overall quality of evidence supporting an improvement in clinical pregnancy and live birth rates using HA-supplemented medium as moderate, while a subsequent RCT did not find a beneficial effect on clinical pregnancy, implantation, and delivery rates. The birth weight however was significantly higher in the HA group than in the conventional ET medium group.21,22 When four criteria are used to categorise this add-on, the use of the HA-supplemented media could be defined as an experimental treatment because only short-term neonatal outcome is available (score 2 for safety). Since further RCT studies are needed to obtain robust conclusions concerning the effectiveness of HA supplemented media, HFEA assigned the amber colour (Table 1).
ENDOMETRIAL SCRATCHING
The induction of endometrial injury to stimulate an inflammatory response and increase angiogenesis has been proposed as a strategy to improve embryo implantation. It is important to underline that the exact biological mechanisms behind the possible beneficial effect on implantation remain unclear. Risks related to this procedure are very rare and include infection and uterine perforation. Multiple RCT and meta-analyses have been performed measuring different effects.23,24 Studies are of moderate quality at best and the evidence is compromised by the high clinical heterogeneity.23 A very recent RCT showed that the endometrial scratching did not result in a higher rate of live birth compared to no intervention among women undergoing IVF.24
The inconclusive and sometimes contradictory results indicate that no solid conclusions can be drawn. No short or long-term data are available regarding children born after IVF cycle with endometrial-scratch procedure. It is because of these reasons and the score 2 for safety that endometrial scratching is categorised in the Provoost model as part of the group of experimental treatments although HFEA assigned the amber colour (Table 1).
REPRODUCTIVE IMMUNOLOGY TESTS AND TREATMENT
Failure of immune-mediated processes required to establish a maternal immune tolerance has been proposed as a potential cause of infertility. Based on this theory, different tests and therapies have been proposed for recurrent implantation failure and recurrent pregnancy loss patients such as the use of corticosteroids, intravenous immunoglobulins, TNFα antagonists (e.g., adalimumab, infliximab), calcineurin inhibitors (e.g., tacrolimus), and intralipid infusions.25 Conflicting results have been reported concerning the improvement of clinical outcomes via these immunomodulating and immunosuppressive therapies.25 A paucity of safety data indicates that the use of these tests and treatments requires a careful ‘benefit to risk’ evaluation.25 To date, the lack of large RCT, no protocol standardisation related to reproductive immunology, and no reassuring data on safety to support the clinical benefit leaves this topic under investigation. Thus, reproductive immunology tests and treatments can be considered experimental, in agreement with the HFEA opinion for which the red colour was assigned (Table 1).
TIME-LAPSE IMAGING
Emerging as a new technology to guarantee a continuous embryo monitoring, the time-lapse system (TLS) represents one of the potential tools of the IVF laboratory. The possibility of an undisturbed culture associated with the evaluation of morphokinetic parameters linked with an algorithm for embryo selection has the potential to improve live birth rate. Nowadays, RCT and meta-analyses have been performed. The last Cochrane Review reported the quality of evidence ranging from very low to moderate.26 In fact, there are inconclusive data in terms of live birth, miscarriage, stillbirth, or clinical pregnancy to choose between TLS, with or without embryo selection software, and conventional incubation.26 No detrimental effects were observed using TLS on obstetric or perinatal outcomes.27 TLS represents an important innovation in the IVF laboratory with reassuring preclinical outcomes, but today this technology is not more effective than conventional methods for embryo incubation. In conclusion, a lack of published short-term follow-up data on children born after embryo culture with TLS limits the evaluation of this technology. When applying the Provoost model, it can be classified as an experimental treatment because of the score 2 for the safety, although HFEA assign it to the amber category (Table 1).
INTRACYTOPLASMIC MORPHOLOGIC SPERM INJECTION
Sperm morphology assessment using high magnification (over ×6,000) allows identification of sperm abnormalities, such as the presence of nuclear vacuoles. These abnormalities have been suggested to have negative effects on ICSI outcome.28 The integration of intracytoplasmic morphologic sperm injection (IMSI) technology in the ICSI procedure promised to increase pregnancy and/or birth rates in male factor infertility. No additional risks are reported with respect to ICSI and several RCT and meta-analyses have been published in the last decade. No convincing evidence of any significant difference between IMSI and ICSI in terms of pregnancy rate or live birth rate has been reported however.29 These results do not suggest a routine use of this technique in IVF treatment, therefore the HFEA assigned it the red colour (Table 1). Reassuring data regarding safety30 (score 3), relative procedure variability (score 2), and moderate effectiveness (score 2) scoring, categorises IMSI as an innovative treatment by the Provoost model.
ADDITIONAL INFORMATION – SPERM DNA DAMAGE
Sperm DNA integrity represents an important parameter of male gamete quality. Several assays for sperm DNA fragmentation have been used and the type of damage measured with various grades of sensitivity are different, generating high heterogeneity among the studies.31 There is no evidence that endorses the use of one assay over another. An important topic is the management of treatment as a result of this diagnostic test. Different strategies have been proposed such as lifestyle modifications, infection control, or oral antioxidant therapy, but there is not enough evidence of effectiveness for the treatment(s).1 This culminates in the question of whether sperm DNA testing has a role during andrological examination. In 2013, the Practice Committee of the American Society for Reproductive Medicine (ASRM) concluded that sperm DNA testing cannot be recommended routinely for clinical use based on the insufficient evidence.32 Because DNA fragmentation is a diagnostic, noninvasive test and not a treatment, no traffic light has been assigned from HFEA to sperm DNA damage, and likewise the Provoost model was not applied (Table 1).
CONCLUSION
Medical assisted reproduction is in continuous progress and development. It is advantageous for patients to have access to the most successful and safest treatments with the highest outcome. However, the emotional race against the fertility clock to conceive and give birth to a healthy child makes patients vulnerable to the application of less effective techniques, sometimes even without reassuring safety data. Various supplementary treatments have found their way into the clinical practice of assisted reproduction without being critically assessed on their effectiveness. The HFEA has categorised 12 ‘add-ons’ according to the traffic-light system to inform patients on the effectiveness of these supplementary treatments. HFEA concludes that rigorous RCT and robust data on safety are often lacking and therefore the use of add-ons should be reconsidered to avoid false hope and unnecessary costs for the patient.
Here, the add-ons were categorised by the Provoost model: a framework using a 3-stage classification of treatments. Most of the add-ons were categorised in agreement with the HFEA classification; the red traffic light corresponded to the class of the experimental treatment, whereas the amber colour aligned with the innovative category. For the PGT-A 2.0 embryo glue, the endometrial scratching, and the time lapse imaging, HFEA assigned an amber light, whereas the application of the Provoost model directed these four to the class of the experimental treatment.
A possible explanation for the lack of concordance between the HFEA traffic light rating system and the Proovost model for some treatments could be that some RCT were not available when the HFEA had released its model; for instance, the RCT on endometrial scratching.24
The classification of treatments based on predefined criteria as proposed by Provoost et al.3 is useful because it gives a structured methodology to evaluate the literature used as input for the model. Although, these evaluations are subject to bias and discussion. Models and frameworks can help; however, every instrument has its pro and cons. The model proposed by Provoost et al. takes into account four criteria to move a treatment onto the continuum.3 Because many studies report only on perinatal outcome, and not on mid-term safety of the children, this requirement for innovative treatment was not always met, hence the resulting score in the Provoost model.
Aside from the patients’ information and these add-on classifications in IVF treatments, it is important that the correct follow-up studies are designed, and that long-term outcome data are published on these treatments. Nowadays, there is a substantial gap in knowledge regarding the long-term health of children born after ART and the effects of the introduction of new ART techniques on offspring must be monitored.33 Especially for an innovative treatment, IVF centres should make a commitment to closely monitor their practice by conducting follow-up studies. Only when these data are reported can well-informed clinics provide the most effective and safest care for their patients.








