Abstract
Congenital adrenal hyperplasia (CAH) refers to a group of disorders that are associated with defective adrenal steroidogenesis, the most common of which is 21-hydroxylase deficiency. The advent of neonatal screening, molecular genetics, and glucocorticoid and mineralocorticoid replacement has vastly improved the diagnosis and treatment of CAH; therefore, most infants and children with CAH successfully transition into adulthood. Several quality-of-life issues emanate from this transition, of which reproduction and fertility are notable. In this review, the authors appraise the effects of elevated androgens in CAH on the anatomic, hormonal, and psychosocial aspects of reproductive function. These CAH-associated alterations in reproductive anatomy or endocrine function can impair natural fertility, most often depending on the severity of CAH. In addition to assessing the fertility rates of women with CAH attempting natural conception, as well as those requiring assisted reproductive treatments, the authors also review data pertaining to the mode of delivery and pregnancy outcomes in these women. Finally, the importance of reproductive and preconception counselling in women with CAH attempting conception is briefly discussed.
INTRODUCTION
Congenital adrenal hyperplasia (CAH) is an autosomal recessive disorder that is caused by defective steroidogenesis in the adrenal cortex.1 Although frequently referred to as a single entity, CAH comprises different variants depending on the enzyme defect involved in adrenal steroidogenesis. Of all the CAH cases, >90% are caused by 21-hydroxylase deficiency.2 These CAH cases can be further subdivided into classic CAH and the milder non-classic CAH.3 The former comprises salt-wasting and simple-virilising forms of CAH. In the most severe salt-wasting form, deficiency of the 21-hydroxylase enzyme results in insufficient production of both aldosterone and cortisol. In contrast, the more moderate simple-virilising form of CAH is characterised by deficient cortisol but normal aldosterone production; thus, salt-wasting does not occur in this form. Both salt-wasting and simple-virilising CAH result in elevated androgens that cause virilisation of the external female genitalia. The non-classic form of CAH is associated with a mild 21-hydroxylase enzyme defect. Consequently, this form of CAH results in only mild elevations of adrenal androgens that do not affect the external genitalia. It is important to note that non-classic CAH is more prevalent than classic CAH, i.e., 1 in 600 versus 1 in 16,000 cases, respectively.4 In fact, the prevalence of non-classic CAH can be as high as 3.7% (4 in 100) in the Ashkenazi Jewish population.5
CAH may be also caused by other enzyme deficiencies, namely 11β-hydroxylase, 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase/17-20 lyase, and cytochrome P450 oxidoreductase deficiency.1 This review will focus on CAH caused by 21-hydroxylase deficiency.
EFFECTS OF CONGENITAL ADRENAL HYPERPLASIA ON REPRODUCTION AND FERTILITY
As adrenal steroid hormones are essential to normal sexual development and reproductive function, deficiencies of these hormones can impact a woman’s reproductive fertility and function.6 Thus, it is not surprising that CAH is the most common genetic disorder of steroidogenesis affecting fertility.6,7 Several anatomic, hormonal, and psychosocial mechanisms have been proposed to explain the effects that CAH may have on normal reproductive function and fertility (Box 1). Many of the mechanisms listed below are not unique to 21-hydroxylase deficiency. Studies have highlighted that women with CAH due to other enzyme defects may have reproductive issues similar to women with 21-hydroxylase deficiency-induced CAH, though with varying presentations.1
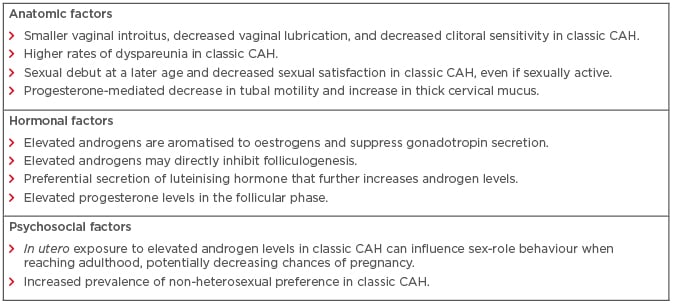
Box 1: Summary of anatomic, hormonal, and psychosocial parameters affecting reproductive fertility and function in women with congenital adrenal hyperplasia.
CAH: congenital adrenal hyperplasia.
Due to the virilising effects of androgens on the external genitalia, women with classic CAH have a smaller vaginal introitus, decreased vaginal lubrication, and decreased clitoral sensitivity, as well as significant dyspareunia.8,9 These factors often contribute to a later sexual debut as well as ongoing anxiety about sexual performance.8,10 This was exemplified by a cross-sectional study of 35 women with classic CAH; the study reported that 37% of women never had vaginal intercourse and that 81% of women experienced pain during intercourse.11 The overall quality of sexual experience in women with CAH also tends to be lower than unaffected women, even after meticulous surgical genitoplasty.12,13 Collectively, these factors result in decreased sexual activity, thereby diminishing the probability of natural conception.
CAH is known to alter the function of the hypothalamic–pituitary–ovarian axis. Potential aetiologies for the alterations to the hypothalamic–pituitary–ovarian axis include elevated androgens, elevated progesterone, expression of 5α-reductase in the ovary, or even a direct glucocorticoid effect.8,14 Initial observations suggested that excess androgens are aromatised to oestrogen, which could suppress gonadotropin secretion.15 Elevated androgen levels were also thought to inhibit folliculogenesis, albeit in rat models.16 However, recent evidence has indicated that androgen excess impairs hypothalamic sensitivity to progesterone.8 This causes increased gonadotropin-releasing hormone pulse frequency, resulting in a preferential secretion of luteinising hormone (LH). The hypersecretion of LH increases ovarian androgen production, which further potentiates and intensifies the effects of adrenal androgens.8,17 Hypersecretion of LH is also noted in patients with polycystic ovarian syndrome (PCOS).18,19 Therefore, there is considerable overlap between the manifestations of CAH and PCOS. In fact, patients with classic or non-classic CAH may often present with acne, hirsutism, alopecia, and oligomenorrhoea or amenorrhoea, which are pathognomonic of PCOS.20-22 Furthermore, polycystic ovaries can be observed in approximately 40% of all women with non-classic CAH.21 The association of chronic anovulation and irregular menstrual cycles with CAH can further decrease the fecundity of women with CAH.
Elevated progesterone levels can be detrimental to the reproductive potential of women with CAH. In contrast to the biphasic pattern of progesterone secretion in the follicular and luteal phases of unaffected women, progesterone levels are consistently elevated in women with CAH.6,23 Elevated progesterone not only alters gonadotropin-releasing hormone pulse frequency but also decreases tubal motility, thickens cervical mucus, and diminishes endometrial receptivity.24,25
The issue of fertility is also closely associated with psychosexual development.2 In addition to the effects on the external genitalia of women with classic CAH, in utero exposure to elevated androgens may influence sex-role behaviour.26,27 For example, affected women are reported to exhibit increased male-type behaviour during childhood, as exemplified by toy preferences and aggressiveness.26,28 While most women with CAH are heterosexual with a female sexual identity,2 there is an increased prevalence of non-heterosexual preference in women with classic CAH, which correlates with disease severity.29,30 Studies have also suggested that women with classic CAH may avoid heterosexual vaginal intercourse, especially in the presence of a small vaginal introitus,31 thus contributing to apparent reduced fertility.
OVARIAN RESERVE IN WOMEN WITH CONGENITAL ADRENAL HYPERPLASIA
Ovarian reserve is a commonly used term to describe the reproductive potential in women, based on the quantity or reserve of remaining oocytes.32 Different clinical markers, including antral follicle counts, ovarian volumes, serum levels of follicle-stimulating hormone, and anti-Müllerian hormone (AMH) on cycle Day 2, have been used in routine clinical practice to determine a woman’s ovarian reserve. A recent prospective study compared ovarian volumes and AMH levels between 33 CAH patients and 33 age-matched controls.33 There was a non-significant trend towards larger ovarian volumes in the CAH group (4.4 mL; range: 1.3–10.8 mL) when compared to controls (2.8 mL; range: 0.6–10.8 mL). In contrast, there was no significant difference in median serum AMH levels between CAH patients (11.0 pmol/L; range: 1–36 pmol/L) and controls (13.0 pmol/L; range: 1–45 pmol/L). Of note, median serum AMH levels were comparable in women with classic or non-classic CAH. Furthermore, there was no association between ovarian volumes, AMH levels, and androgen levels in CAH patients.
FERTILITY IN WOMEN WITH CONGENITAL ADRENAL HYPERPLASIA
Most data in the medical literature focus on the fertility of women with 21-hydroxylase deficiency; however, there have also been case reports and case series of fertility outcomes in women with CAH due to rarer mutations.34-38
Fertility in Women with Classic Congenital Adrenal Hyperplasia
The fertility potential of women with classic CAH generally mirrors the severity of their underlying disease.6 Women with severe disease have significantly decreased sexual activity and overlap with PCOS-type anovulatory menstrual cycles, which cumulatively leads to decreased fertility rates.39 It is important to note that women with classic CAH invariably require glucocorticoid and mineralocorticoid replacement to induce ovulatory menstrual cycles. Thus, natural conception in the absence of treatment is exceedingly rare. In fact, the earliest pregnancies in classic CAH patients coincided with the introduction of pharmacologic cortisol.40
Women with classic CAH can conceive while on routine maintenance therapy, and it is estimated that 80% and 60% of women with simple-virilising and salt-wasting forms of CAH, respectively, are fertile.41 Most women who are compliant with maintenance therapy have ovulation rates as high as 40%.42 However, some women require higher doses of glucocorticoids to adequately suppress adrenal androgens and initiate ovulatory menstrual cycles.6 Despite these factors, women with classic CAH may have lower pregnancy rates when compared to unaffected age-matched controls.43,44 In one Swedish study, 62 women with classic CAH were compared to 62 age-matched controls.45 There was no difference in the age at menarche or first pregnancy between the groups. The frequency of irregular menstrual cycles and prior use of hormonal contraception was also comparable. However, the number of clinical pregnancies (31 versus 76) and live births (25 versus 54) was significantly lower among women with CAH. All children in the study had normal birth weight without any major malformations. Another study of 25 women with classic CAH in the UK reported similar pregnancy rates in CAH women (21/23, 91.3%) and age-matched controls in the normal population.46 Yet, the fertility rate, defined as live birth rate per woman, was lower in the CAH group (0.25 per woman) when compared to the general population (1.8 per woman). An association has also been reported between CYP21A2 mutation severity and fertility rates: no live births in the null group, 3% in the I2 splice group, and 33% in the Ile172Asn group.45
Fertility in Women with Non-Classic Congenital Adrenal Hyperplasia
While women with non-classic CAH are the least likely to be infertile, these women are more likely to be seen by medical providers due to the increased frequency of non-classic CAH over classic CAH and symptoms such as hirsutism or menstrual cycle irregularities.6 In one study of 190 women with non-classic CAH, only 20 patients consulted for infertility.47 Of the 190 women, 95 desired pregnancy, of which 85 conceived successfully. In these 85 women, 187 pregnancies occurred, resulting in the birth of 141 children in 82 of them. Overall, 57.2% of pregnancies occurred naturally without any treatment, 41.2% of pregnancies with hydrocortisone treatment, and 1.6% of pregnancies with ovulation induction agents. Of note, the rate of miscarriage was lower (6.5%) in women receiving glucocorticoid treatment compared to those without (26.3%). Of the 141 live born children, only 2 (1.5%) were born with classic CAH. A separate study of 203 pregnancies among 101 women with non-classic CAH reported a higher frequency of natural conception (68%) when compared to conception after glucocorticoid treatment (32%).48 Similar to the results of the Bidet et al.47 study, the spontaneous miscarriage rate was lower in the treated group (6.2% versus 25.4%). The rates of classic and non-classic CAH among the live born were 2.5% and 14.8%, respectively.
Fertility Treatments
Many women with classic or non-classic CAH may remain anovulatory despite adequate pharmacologic therapy or may have ovulatory cycles with elevated progesterone levels in the follicular phase.49 In such cases, treatment with prednisolone 2–5 mg three times per day has been reported to decrease circulating progesterone levels during the follicular phase, thereby increasing the likelihood of natural conception.1,46 Laparoscopic bilateral adrenalectomy, though controversial, can be used in select patients with elevated androgen and progesterone levels that are refractory to conventional medical therapy.50 Despite its effectiveness, bilateral adrenalectomy can increase the risk of future adrenal crises in patients with poor medication compliance.51 Patients who still remain anovulatory or who are unable to conceive with the aforementioned treatments can benefit from ovulation induction with clomiphene citrate, aromatase inhibitors, or gonadotropin injections, and adjunctive metformin.52-54 In vitro fertilisation (IVF) with or without preimplantation genetic diagnosis can be used in women if other fertility treatments are ineffective.1,55
As stated earlier, women with CAH may have symptoms that overlap with PCOS. Thus, anovulation and hyperinsulinaemia can be common in women with CAH. Prior studies have revealed that medications such as metformin or inositol, either alone or in combination, can mitigate insulin resistance and increase fecundity by increasing the frequency of spontaneous ovulation.56 Furthermore, when used in IVF, metformin or inositol supplementation has been shown to increase oocyte and embryo quality.57
PREGNANCY OUTCOMES IN WOMEN WITH CONGENITAL ADRENAL HYPERPLASIA
Observational data have suggested that pregnancies in untreated women with classic or non-classic CAH are at a higher risk of spontaneous miscarriage.45,47 However, these findings have not been confirmed in larger populations. Antepartum complications, such as pre-eclampsia and preterm birth, are similar in the CAH and general population. The mode of delivery in women with CAH, particularly those with classic CAH, remains a consistent concern. Studies have posited that early exposure to elevated levels of androgens may cause the maternal pelvis to assume a narrower, android configuration instead of the usual gynecoid pelvis.58 Thus, while vaginal delivery is still possible,59 the risk of labour arrest and subsequent caesarean delivery is high. Caesarean delivery may also be performed electively to prevent perineal trauma in women who have undergone surgical genitoplasty. In the Hagenfeldt et al.45 study of 62 women with CAH, 25 women had children, of which 21 delivered via caesarean delivery, which was most often performed due to a history of prior genital surgery. In 2 patients, caesarean delivery was performed due to cephalo-pelvic disproportion, likely due to the inadequacy of the pelvis in allowing fetal descent.45 Current data also confirm reassuring neonatal outcomes in mothers with CAH. In fact, virilisation of female neonates is rare even in mothers with classic CAH, usually occurring due to poor medication compliance.60,61 The lack of virilisation is attributed to the activity of placental aromatase (i.e., conversion of androgens to oestrogens protects the female fetus from virilisation).61 The long-term physical and intellectual development of children born to CAH mothers has also been normal.44
PRECONCEPTION COUNSELLING
Careful preconception counselling should be undertaken in all women with CAH contemplating pregnancy.49 This counselling should include the risk of CAH transmission based on carrier status and CAH genetics, as well as possible pregnancy complications. Most studies suggest that the carrier frequency for classic CAH is approximately 1 in 62; the carrier frequency for non-classic CAH ranges between 1 in 5 and 1 in 16 depending on the population assessed.62,63 It is particularly important to screen and counsel women with non-classic CAH for severe mutations that are phenotypically silent.49 Data from the Moran et al.48 study of 101 women with non-classic CAH reported a risk of 2.5% and 14.8% of conceiving a child with classic and non-classic CAH, respectively.48 Some investigators have proposed that centres offering assisted reproductive treatment should consider screening infertile patients for CAH given the overlap between CAH, infertility, and PCOS.62 In some cases, infants with classic CAH have been born after IVF to women harbouring CAH mutations that remained undiagnosed prior to treatment.62
Prenatal treatment of CAH also deserves special mention. Administration of dexamethasone before the 9th week of gestation can suppress adrenal androgen production and prevent virilisation of the external female genitalia. However, prenatal treatment of CAH remains controversial. This is primarily due to the potential impact of glucocorticoids on the developing fetal brain and postnatal growth and development.64
CONCLUSION
As highlighted in the current review, CAH can have a profound impact on the synthesis of various adrenal steroids that may result in various reproductive issues. The detrimental effects of CAH on natural conception may occur due to alterations in reproductive anatomy or endocrine function. While patients with CAH have normal ovarian reserve parameters, they frequently require pharmacologic maintenance therapy to induce ovulatory menstrual cycles when attempting conception. Suppression of androgens from the adrenal gland is usually the first therapeutic strategy to achieve spontaneous ovulation and conception. Ovulation induction agents or IVF can be used in CAH patients who are unable to conceive naturally or with anovulatory menstrual cycles despite adequate treatment. To avoid perineal trauma, elective caesarean delivery is performed in women with a history of genitoplasty. Current data indicate reassuring pregnancy outcomes for CAH mothers, as well as long-term developmental and health outcomes of children born to CAH mothers. Finally, careful preconception counselling should be undertaken in CAH patients contemplating pregnancy.








