Abstract
Background: Ruptured ectopic or extrauterine pregnancy (EP) is responsible for 6% of maternal deaths in the first trimester. This review was designed to summarise the diagnostic criteria and treatment modalities of EPs.
Methods: Recent guidelines of the international societies of obstetrics and gynaecology, including the Royal College of Obstetricians and Gynaecologists (RCOG), the American College of Obstetricians and Gynecologists (ACOG), and the European Society of Human Reproduction and Embryology (ESHRE), were reviewed to summarise the diagnostic criteria and treatment modalities of EPs.
Results: A minimum β-human chorionic gonadotropin (β-hCG) rise of ≥35% in 48 hours was suggested to diagnose intrauterine pregnancy. A β-hCG rise <35% in 48 hours has 96.2% positive predictive value, 69.7% negative predictive value, and 80.2% overall accuracy in predicting EPs. The blob sign has >90% positive predictive value in diagnosing EPs in symptomatic females with positive β-hCG and no definite intrauterine gestational sac by transvaginal sonography. The interstitial ectopic pregnancy and cornual pregnancy are two separate entities of EPs. Interstitial line sign has 80% sensitivity and 98% specificity in diagnosing interstitial ectopic pregnancy. A meta-analysis reported 89% overall success rate for methotrexate in treatment of EPs; the multi-dose regimen was significantly more successful than the single-dose regimen.
Conclusion: Institutes and healthcare providers should follow clear guidelines and/or protocols for the management of EPs. Institutes should implement competency-directed training programmes to increase healthcare providers’ skills to diagnose and treat EP variants using different modalities.
INTRODUCTION
Ectopic or extrauterine pregnancy (EP) is a first trimester pregnancy complication that occurs in 1.3–2.4% of all pregnancies.1 Ruptured EP is responsible for 6% of maternal deaths in the first trimester.1,2 The increased rates of assisted reproductive techniques (ARTs), tubal surgeries, and improved diagnostic tools are responsible for increased incidence of EPs.1
A minimum β-human chorionic gonadotropin (β-hCG) rise of ≥35% in 48 hours was suggested to diagnose intrauterine pregnancy (IUP). A β-hCG rise <35% in 48 hours has 96.2% positive predictive value (PPV), 69.7% negative predictive value, and 80.2% overall accuracy in predicting EPs.3 The blob sign has >90% PPV in diagnosing EPs in symptomatic females with positive β-hCG and no definite intrauterine gestational sac (IUGS) by transvaginal sonography (TVS).2
The interstitial ectopic pregnancy (IEP) and cornual pregnancy are two separate entities of EPs. Interstitial line sign has 80% sensitivity and 98% specificity in diagnosing IEP.4-9
The American College of Obstetricians and Gynecologists (ACOG) reported spontaneous resolution of EPs in 88% of patients at initial β-hCG <200 mIU/mL following expectant managment.10
A meta-analysis reported an 89% overall success rate for methotrexate (MTX) in treatment of EPs; the multi-dose regimen was significantly more successful than the single-dose regimen but caused more side effects.11 Despite the available diagnostic tools, delayed diagnosis and unreliable follow-up make ruptured EPs a daily practice in obstetrics and gynaecology. This literature review was designed to summarise the diagnostic criteria and treatment modalities of EPs.
DIAGNOSTIC CRITERIA AND TREATMENT MODALITIES OF ECTOPIC PREGNANCIES
EPs occur following implantation of the fertilised ovum outside the normal uterine cavity.
Classification of Extrauterine Pregnancies
- Tubal EPs. These constitute >90% of EPs. In 80% of cases, tubal EPs occur in the ampullary region of the fallopian tube (FT). It may occur in other parts of the FT, including isthmus, fimbria, or the interstitial portion.
- Non-tubal EPs. These constitute <10% of EPs and may be cervical, ovarian, intramural, or abdominal.
- Heterotopic pregnancies (HPs).
- Caesarean section (CS) scar pregnancies (CSSP).
Incidence of Extrauterine Pregnancies
- Tubal EPs occur in 1.3–2.4% of all pregnancies.1 IEPs account for 4% of EPs.
- Non-tubal EPs:
- Cervical EPs occur in 1:2,000–1:18,000 pregnancies. The incidence of ovarian EP is 2% and is the most common type of non-tubal EP.12
- Intramural EP (pregnancies within the myometrium) account for 1% of EPs. Abdominal pregnancy (AP) accounts for 1.3% of EPs.13
- HPs occur in 1:4,000–1:30,000 in the general population.
- CSSP incidence rate increased following the increased caesarean section rates, and occurs in 1:1,800–1:2,216 pregnancies.2
Taran et al.1 explained the rising incidence of EPs by the increased rates of ARTs, tubal surgeries, and improved diagnostic tools.1 Perkins et al.14 reported that the incidence of EPs following ARTs has decreased over time but the risk of EP increased following multiple embryo transfers during ARTs.14 In addition, the ACOG reported the multiple embryo transfers and tubal factors of infertility as risk factors for EPs following ARTs.10 Ruptured EP is responsible for 6% of maternal deaths in the first trimester.1,2 A review of mortality in ART-associated EPs reported a mortality rate of 31.9 deaths per 100,000 pregnancies.15
AETIOLOGY OF EXTRAUTERINE PREGNANCIES
The FT is the site of oocyte fertilisation. The migration of the fertilised oocyte to the uterine cavity for implantation is mediated by the FT smooth muscles and cilia. FT inflammation and/or dysfunction are implicated in oocyte retention and subsequent EPs.2
Risk Factors for Tubal Extrauterine Pregnancies
Up to 50% of females diagnosed with EPs have no identifiable risk factors; however, several risk factors have been associated with EPs.1 High-risk factors (odds ratio [OR]: >4.0) include prior tubal surgery, prior EP, sterilisation, and intrauterine device (IUD) users.1 Moderate risk factors (OR: >2.0) include infertility, current or prior pelvic inflammatory disease, smoking, and more than one sexual partner.1 Mild risk factors (OR: <2.0) include age >40 years.1 IUD use decreases the overall pregnancy rates and prevents intrauterine implantation, with subsequent higher incidence of EPs in females conceiving with an IUD in place compared to the general population.11
Risk Factors for Non-tubal Extrauterine Pregnancies
Previous dilation and curettage are risk factors of cervical EPs.Myometrial injury during curettage, myomectomy, or CS is a risk factor for intramural EPs.Pelvic inflammatory disease, ARTs, and endometriosis are risk factors for APs.1
Risk Factors for Caesarean Section Scar Pregnancies
Although the European Society of Human Reproduction and Embryology (ESHRE) classified CSSP as one of the uterine EPs,15 other authors classify CSSP as an IUP because it may result in live offspring if not terminated.16 CSSPs occur following implantation of the fertilised oocyte over the previous CS or hysterotomy scars.16 The number of scars is not a risk factor for CSSP, but it occurs following elective CS, which is explained by the impaired healing of an unprepared lower uterine segment.2
Clinical Presentation of Extrauterine Pregnancies
The triad of secondary amenorrhoea, first trimester spotting, and pelvic pain may occur with EPs as well as intact IUPs and early miscarriages. Further suggestive manifestations of EPs include pain on movement of the cervix, acute abdominal pain radiating to the shoulder(s), abdominal guarding, hypovolaemic shock, and syncope. Differential diagnosis of EPs includes complicated ovarian or adnexal cyst, tubo-ovarian abscess, appendicitis, and ovarian hyperstimulation syndrome.1
DIAGNOSIS OF EXTRAUTERINE PREGNANCIES
β-human Chorionic Gonadotropin
Suspicion of an EP begins after a positive pregnancy test and with the absence of definite IUGS by ultrasound; i.e., pregnancy of unknown location (PUL). Definite IUGS means IUGS with yolk sac and/or embryo.1,13 The diagnosis of PUL will be changed to IUP in approximately 30% of cases after appearance of definite IUGS by ultrasound, while the majority of PUL (50–70%) will be changed to either miscarriages or EPs. β-hCG is produced by the syncytiotrophoblasts, detected in the blood by the second week of pregnancy, and its measurement is crucial to clarify pregnancy location and prognosis.2 A single β-hCG assay is insufficient to detect early pregnancy prognosis, and serial β-hCG are commonly used to monitor early pregnancies.3
The recommendations of β-hCG rise came from retrospective review of PUL, which suggested a minimum β-hCG rise of ≥35% in 48 hours to diagnose IUP.3 A β-hCG rise <35% in 48 hours has 96.2% PPV, 69.7% negative predictive value, and 80.2% overall accuracy in predicting EPs.3 EPs are generally associated with a rise in β-hCG by no more than 66%, or a fall by no more than 13% from the baseline level, in 48 hours. The combination of β-hCG ratio lying within this range, along with initial β-hCG >1,500 mIU/mL in the absence of definite IUGS, has 92% sensitivity and 84% specificity in predicting EPs.17
Serum Progesterone
A meta-analysis concluded that a serum progesterone level of <10 ng/mL predicts non-viable pregnancy with 66.5% sensitivity and 96.3% specificity.18 Serum progesterone is not useful in predicting EPs and cannot differentiate EPs from early miscarriages.19
Discriminatory Zone
The discriminatory zone is the serum β-hCG level at which definite IUGS can be seen by the TVS, confirming the diagnosis of IUP (≥1,000–2,000 mIU/mL).2 Despite the discriminatory zone, a review of PUL reported visualisation of IUGS confirming the diagnosis of IUPs in nine females with β-hCG >2,000 mIU/mL.20 In multiple pregnancies, definite IUGS(s) may be seen at β-hCG value higher than the discriminatory zone identified for singleton IUP.2
A positive pregnancy test in the absence of definite IUGS should be considered EP until proven otherwise. The risk of EP is much reduced (but not zero) after visualisation of IUGS because of the possibility of HP.2
Additional Ultrasound Findings
Additional ultrasound findings may be useful to increase the suspicion for EPs. IUPs have significantly higher endometrial thickness than miscarriages or EPs (17 mm versus 12 mm, respectively).21 In 20% of tubal EPs, a collection of fluid within the uterine cavity could be seen, referred to as the pseudosac (pseudosac is not diagnostic for tubal EPs).21 A small amount of fluid in the Pouch of Douglas may be found in both IUPs and EPs, while large amounts of free fluid, particularly in the Morrison’s pouch, may indicate haemoperitoneum in ruptured EPs.2 Visualisation of an adnexal gestational sac (GS) with yolk sac and/or embryo as an echogenic periphery and non-echogenic interior (bagel sign) with circumferential Doppler flow without definite IUGS is highly suggestive of EP.1 If the visualised, suspicious GS is round, echogenic, and moves separately from the ovary, this is considered the ‘blob sign’. The blob sign has >90% PPV in diagnosing EPs in symptomatic females with positive β-hCG and no definite IUGS by TVS.2
A systematic review concluded that 88% of tubal EPs were diagnosed by the combination of an absent IUGS with an adnexal mass.2 Ultrasound criteria of IEPs occur following implantation of the fertilised oocytes in the interstitial portion of the FT when it traverses through the uterine muscular wall (1–2 cm) to enter the cavity, and is a separate entity from the cornual pregnancy. Cornual pregnancy describes pregnancy in the rudimentary horn of a uterus with Müllerian anomaly.4-9 The ultrasound criteria of the IEPs include an empty uterus with an eccentric GS seen separate from the endometrium, and the GS >1 cm away from the most lateral edge of the uterine cavity and surrounded by <5 mm myometrium in all imaging planes. Interstitial line sign has 80% sensitivity and 98% specificity in diagnosing IEPs. Interstitial line sign is an echogenic line extending from the GS to the endometrium cavity, representing the interstitial portion of the FT.4-9
In the absence of definite IUGS, non-tubal EPs can be suspected by specific ultrasound criteria. Cervical EPs can be identified by an empty uterus, a soft and ballooned cervix (barrel-shaped cervix), the GS lying within the cervical canal below the closed internal cervical os with evident blood flow around the GS detected by the colour Doppler, or absence of the sliding sign.13,23 The sliding sign is used to differentiate the cervical EPs from miscarriages within the cervical canal. When pressure is applied on the cervix by the transvaginal probe, the GS slides against the endocervical canal in miscarriage but it does not in cervical EPs.13,23
There are no specific ultrasound criteria for the ovarian EPs.12 Ovarian EPs are suspected by an empty uterus, a wide echogenic ring with internal anechoic area seen in the ovary, evident blood flow surrounding the echogenic ring detected by colour Doppler, and ovarian EP movement with the movement of the ovary when pressure is applied by the transvaginal probe.2 Ruptured ovarian EP could be seen by ultrasound as a complex adnexal mass with free fluid in the Pouch of Douglas.12 It may be difficult to differentiate an ovarian EP from an ovarian cyst, and definite diagnosis can be confirmed at laparoscopy.24
AP can be either primary, following implantation of the fertilised oocyte into the peritoneal surface (rare), or secondary, following ruptured tubal EP or tubal abortion. The ultrasound suggestive criteria of APs include an empty uterus, lack of evidence of dilated FT or complex adnexal mass, a gestational cavity proximal to the anterior abdominal wall and surrounded by bowel loops, and no myometrium separating the gestational cavity from the urinary bladder.25
The diagnosis for primary AP diagnosed with laparotomy using the Studdiford’s Criteria includes healthy tubes and ovaries with no evidence of recent or remote injury, no evidence of utero-placental fistula, and recent pregnancy related exclusively to the peritoneal surface.26
Ultrasound criteria of CSSPs include an empty uterus with an empty, closed endo-cervical canal, location of the GS in the lower anterior quadrant of the uterus below the bladder close to the internal cervical os and previous scar(s) with yolk sac and/or embryo, a thin myometrial layer between the GS and urinary bladder, and numerous blood vessels and arterio-venous malformation around the GS.16
Laparoscopy is the gold standard for surgical treatment of EPs. Its role in diagnosing EPs decreased after the use of TVS and β-hCG.14
TREATMENT OF TUBAL EXTRAUTERINE PREGNANCY
Expectant Management
The ACOG reported spontaneous resolution of EPs in 88% of patients at initial β-hCG <200 mIU/ml following expectant management.10 In addition, a retrospective study, reported 88% success rate following expectant management for EPs at initial β-hCG <200 mIU/ml, while the success rate was 25% success rate at initial β-hCG >2,000 mIU/l27 (Table 1). The Royal College of Obstetricians and Gynaecologists (RCOG) recommends expectant management for tubal EPs at initial β-hCG <1,500 mIU/mL in clinically stable patients with decreasing β-hCG.13
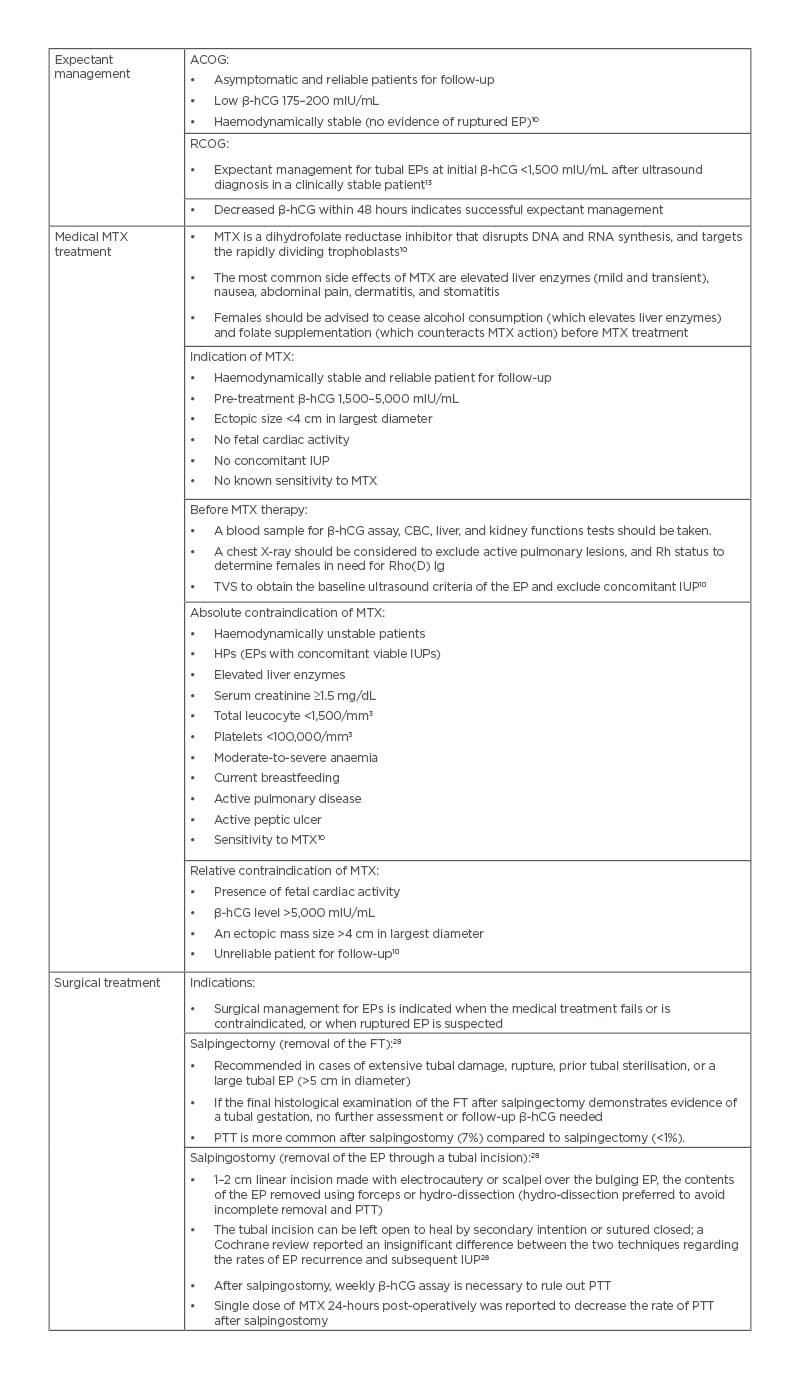
Table 1: Treatment of tubal ectopic pregnancies.
ACOG: American College of Obstetricians and Gynecologists; β-hCG: β-human chorionic gonadotropin; CBC: complete blood count; EP: ectopic pregnancy; FT: fallopian tube; HP: heterotropic pregnancy; IUP: intrauterine pregnancy; MTX: methotrexate; PTT: persistent trophoblastic tissue; RCOG: Royal College of Obstetricians and Gynaecologists; Rh: rhesus; TVS: transvaginal sonography.
Medical Treatment
Medical treatment of EPs using MTX is more cost-effective than surgical treatment, with similar success rates29 (Tables 1 and 2).
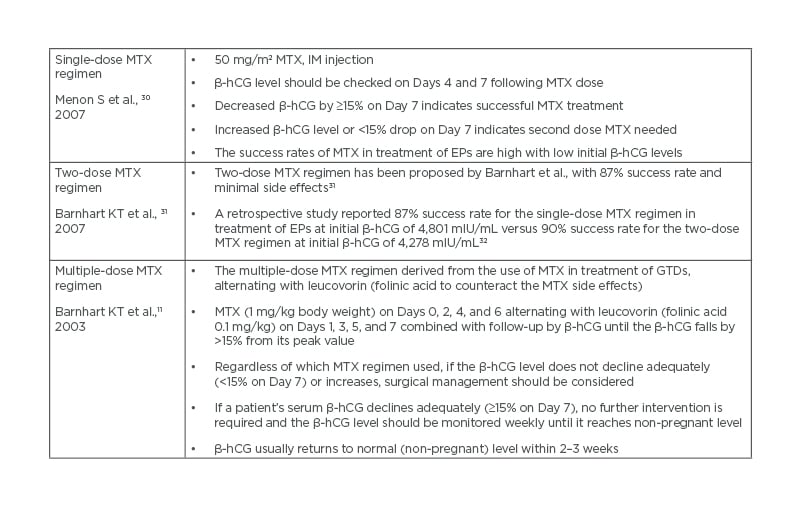
Table 2: Systemic methotrexate regimens.
β-hCG: β-human chorionic gonadotropin; GTD: gestational trophoblastic disease; IM: intramuscular; MTX: methotrexate.
Systemic methotrexate regimens
A systematic review showed that the success rate of single-dose MTX was 94.4% when the initial β-hCG was between 1,000 and 1,999 mIU/mL, and was 81.1% when the initial β-hCG was between 10,000 and 150,000 mIU/mL.30 Two-dose MTX regimen has been proposed by Barnhart et al.31 with 87% success rate.A retrospective study reported 87% success rate for the single-dose MTX regimen at initial β-hCG of 4,801 mIU/mL versus 90% success rate for the two-dose MTX regimen at initial β-hCG of 4,278 mIU/mL.32
A meta-analysis reported 89% overall success rate for multiple-dose MTX in treatment of EPs; the multi-dose regimen was significantly more successful than the single-dose regimen (93% versus 88%) but caused more side effects.11
Surgical Treatment
Laparoscopy is the gold standard for surgical treatment of EPs for fast recovery, minimal hospital stays, and cost.1,2 The surgical methods used for treatment of tubal EPs are salpingectomy or salpingostomy (Table 1).28 The decision of salpingectomy or salpingostomy depends on the contralateral FT status and desired future fertility.14
TREATMENT OF INTERSTITIAL ECTOPIC PREGNANCY
Treatment of IEPs is reliant on gestational age at diagnosis, whether the IEP is intact or ruptured, and desired future fertility. The non-surgical options can be used with intact IEP, while a ruptured IEP is a medical emergency that requires surgery.
Non-surgical Treatment
Non-surgical treatment includes expectant management, systemic MTX, and local injections33,34 (Table 3). The risks of non-surgical treatment for IEPs include subsequent rupture and life-threatening haemorrhage.
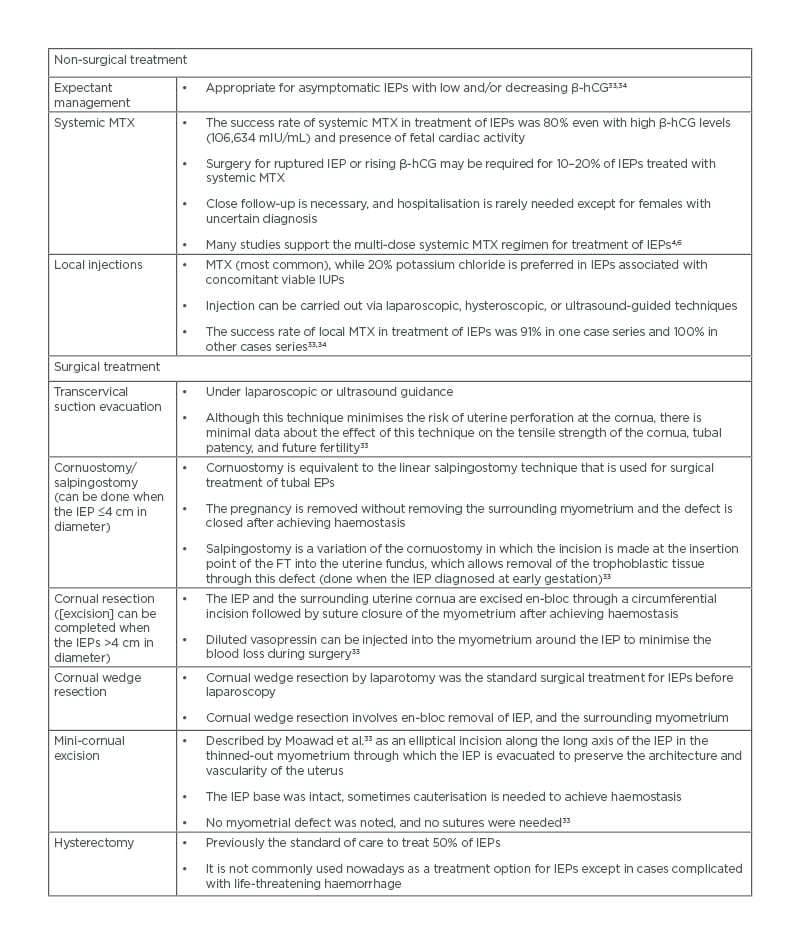
Table 3: Treatment of interstitial ectopic pregnancies.
β-hCG: β-human chorionic gonadotropin; EP: ectopic pregnancy; FT: fallopian tube; IEP: interstitial ectopic pregnancy; IUP: intrauterine pregnancy; MTX: methotrexate.
Surgical Treatment
Surgical treatment is indicated with ruptured IEPs and when non-surgical treatment fails or is not feasible. Laparotomy has previously been the traditional route for surgical treatment of IEPs, particularly ruptured IEPs. Laparoscopic approach is now commonly used for surgical treatment of intact or even ruptured IEPs33 (Table 3).
TREATMENT OF NON-TUBAL ECTOPIC PREGNANCY
Cervical Ectopic Pregnancy
Cervical EPs may be managed medically, surgically, or by combined approaches. The use of systemic and/or local MTX has been described in case reports of cervical EPs.35 An 87% success rate was reported in a series of cervical EPs treated with local MTX and additional potassium chloride injections.36
A success rate of 61.5% was reported in a series of cervical EPs treated with systemic MTX.37 Gestational age >9 weeks, β-hCG >10,000 mIU/mL, positive fetal cardiac activity, and crown–rump length >10 mm were associated with high failure rates of MTX in treatment of cervical EPs.13
Dilation and curettage is not recommended as first-line treatment because of bleeding risks (40% hysterectomy rate reported following dilation and curettage for cervical EPs).2 Placement of cervical sutures, intracervical balloon tamponade, and/or pre-operative feticide injection were reported to minimise the bleeding risks during the management of cervical EPs.38 Uterine artery embolisation has been reported as a prophylactic measure prior to cervical EP treatment or as an emergency treatment.39
Ovarian Ectopic Pregnancy Treatment
Ovarian EP treatment is primarily surgical, and laparoscopy is the gold standard for haemodynamically stable ovarian EPs.12,13 Resection of the ovarian EP and conservative ovarian surgery are usually the main surgical objectives, particularly in females desiring future fertility.12,13
Successful treatment of ovarian EPs using systemic MTX at initial β-hCG up to 5,201 mIU/mL, as well as local MTX injection at initial β-hCG up to 12,075 mIU/mL, has been reported.40,41
Intramural Ectopic Pregnancy Treatment
Intramural EP treatment is dependent on the patient’s condition at the time of diagnosis. Reported cases of intramural EPs in the literature have been managed surgically and sometimes with hysterectomy following ruptured intramural EP.2 Recent reports described the laparoscopic treatment of intramural EPs.42 In clinically stable patients, medical treatment of intramural EPs using systemic MTX was successful even with high β-hCG up to 25,140 mIU/mL.43 Successful management of intramural EPs using local MTX, potassium chloride injections at initial β-hCG of 74,872 mIU/mL,44 as well as uterine artery embolisation at initial β-hCG level of 12,250 mIU/mL,45 have been reported.
Abdominal Pregnancy Treatment
Advanced APs should be managed by laparotomy and a multi-disciplinary team.26 After delivery of the fetus, the placenta should be left in situ if attached by major vessels or vital structures, followed by multi-dose systemic MTX, antibiotics, and follow-up. Attempts to remove the placenta may precipitate severe haemorrhage.26 Pre-operative MRI defines the placental implantation site.26 Intra-operative massive bleeding from the placental site can be controlled by interlocking sutures and/or packing. Packing and/or retained in situ placenta associated with risks of ileus, peritonitis, and abscess formation may necessitate a second-look laparotomy.26 A meta-analysis reported high rates of blood transfusion with hepatic (46%) and retroperitoneal (40%) APs.46
TREATMENT OF HETEROTOPIC PREGNANCIES
A review of the literature showed that most HPs (72.5%) occur in the FT, while seven cases were cervical and three cases were CSSPs.47
Tubal Heterotopic Pregnancies
Medical treatments of tubal HPs include local potassium chloride or hyperosmolar glucose injections. More than 50% of the tubal HPs managed with local potassium chloride injections require subsequent salpingectomy.48 Treatment of HPs with local and/or systemic MTX is contraindicated in the presence of viable IUP.2,13 Surgical management has been described more frequently with tubal HPs because most of the cases presented with tubal rupture.49 Salpingectomy is preferable to salpingostomy in tubal HPs because the persistent trophoblastic tissue cannot be monitored in presence of ongoing IUP.2
Expectant management, hyperosmolar glucose injections, and cornual resection have been reported for management of interstitial HPs with successful live births.50
Non-tubal Heterotopic Pregnancies
Expectant management, local injections (potassium chloride or hyperosmolar glucose), suction curettage, or hysteroscopic resection have been reported for management of cervical HPs with successful live birth. A live birth rate of 80% was reported in a series of cervical HPs for which cervical cerclage was used to minimise the bleeding after the intervention.51
At the time of writing, three cases of live births have been reported after local potassium chloride injection in APs.52 Local hyperosmolar glucose injection and laparoscopic wedge resection were reported for treatment of ovarian HPs with successful live birth.53 Surgical intervention of ovarian HPs carries the risk of interrupting the hormonal support for the co-existing IUP from the corpus luteum.
TREATMENT OF CAESAREAN SECTION SCAR PREGNANCIES
CSSP is a type of IUP, and it may result in a live offspring if not terminated.16 To date, there have been 27 live births reported following conservative management of CSSPs.13 For CSSPs with no yolk sac, and/or fetal cardiac activity, the ultrasound and biochemical follow-up are sufficient until the serum β-hCG returns to non-pregnant levels with or without MTX.16 For CSSPs with yolk sac and/or fetal cardiac activity, there are two treatment options: termination or continuation of the CSSP. Females who decide to continue the pregnancy should be counselled regarding the risks of haemorrhage, uterine rupture, morbid adherent placenta, and emergency hysterectomy.16 To date, there have been 22 emergency hysterectomies reported for life-threatening haemorrhage and morbid adherent placenta following CSSPs.13
Termination of Caesarean Section Scar Pregnancies
Termination of CSSPs should be individualised based on the patient’s age, desired future fertility, and clinician’s experience.
Surgical approaches
Suction aspiration is the traditional choice for surgical approach but it exposes vessels to injury and is associated with major bleeding that may necessitate life-saving hysterectomy. Insertion of a Foley’s catheter at the CSSP site, inflated with saline as compression tamponade, is a potentially useful approach, which can be combined with ultrasound-guided suction aspiration.16 Traditional dilation and curettage is often complicated by haemorrhage (76% required further treatment, and 14% required hysterectomy in a series of CSSPs).54
Hysteroscopic bipolar resection has been reported for CSSP management at initial β-hCG up to 28,333 mIU/mL.55 Hysteroscopic resection is not preferred for CSSP management when the residual myometrium is <3 mm because of uterine perforation and bladder injury risks. Trans-abdominal excision of CSSPs (laparotomy or laparoscopy) allows revision of the lower uterine segment and reduces recurrence risks. Laparotomy is indicated in CSSPs if complicated by life-threatening haemorrhage or suspected uterine rupture.
Local injections
Ultrasound-guided local MTX or potassium chloride injection is the most effective treatment for CSSPs between 6 and 8 weeks; it stops the cardiac activity immediately and should be considered when future fertility desired.16
Systemic Methotrexate Regimens
Several authors have supported the multi-dose systemic MTX regimen for CSSP treatment combined with ultrasound and β-hCG follow-up until the β-hCG returns to non-pregnant levels. Persistent trophoblastic tissue may occur following medical and/or surgical treatments of CSSPs except for hysterectomy.16
PERSPECTIVES AND CONCLUSION
Recurrence of Extrauterine Pregnancies
The recurrence of EPs is not affected by the treatment modality. The ESEP trial showed that the EP recurrence was similar following salpingostomy or salpingectomy (8% and 5%, respectively).56 The reported recurrence rate following prior IEPs was 9.4%.57 One case of recurrent cervical EP reported in a series of cervical EPs was treated with different modalities.58 The data are insufficient to comment on the recurrence rates following prior ovarian, intramural EPs, or APs.
Future Fertility After Extrauterine Pregnancies
Conception is not recommended for 3 months after MTX therapy. IUP rates are similar with no significant difference following salpingostomy or salpingectomy in the ESEP and DEMETER trials.56,59 In addition to this, IUP rates within 2 years after prior tubal EP were similar among salpingectomy (67%), salpingostomy (76%), and medical treatment (76%) in a population-based study.60 Institutes and healthcare providers should follow clear guidelines and protocols for management of EPs. Institutes should implement competency-directed training programmes to increase healthcare providers’ skills to diagnose and treat EP variants using different modalities.







