In this first approach study, couples with a heavy infertility background, between 3 and 9 miscarriages and/or 3–5 failures of assisted reproductive technologies (ART), were tested for the presence of the MTHFR C677T isoform. MTHFR isoforms lead to a weakened methylation ability and a higher sensitivity to oxidative stress, leading to a decreased sperm quality.1,2
In male partners studied, no effect on sperm DNA fragmentation was observed, either for the heterozygous or homozygous patients. However, the isoform was responsible for nucleus decondensation (sperm decondensation index [SDI]) when compared to the control group (n=1,400). SDI was more important for the homozygous patients (HMZ, n=18) than for the heterozygous patients (HTZ, n=70; p<0.05) (Figure 1).
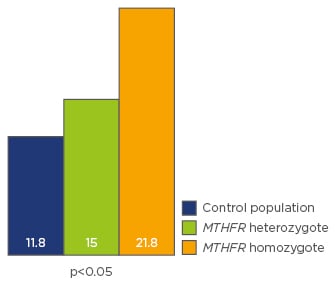
Figure 1: MTHFR C677T isoform and sperm nucleus decondensation.
The numbers represent mean decondensation indexes according to the genetic status.
Nucleus decondensation is a faulty compaction of the nucleus. It is known to induce early embryo developmental arrest,3 as a result of the oocyte being poorly equipped to deal with nucleus tertiary structure anomalies.4
Of the 49 couples studied, 7 (14%) were wild type (WT) X WT and did not participate in the clinical study. Thirteen (26.5%) were HTZ X HTZ, and 12 (24.5%) were HTZ X HMZ. Two couples (4%) were HMZ X HMZ. Patients carrying this isoform have a weak capacity to metabolise folic acid, which impairs the one carbon cycle (1-CC, Figure 2), due to the active compound normally synthesised (5-methyltetrahydrofolate [5-MTHF]) being poorly formed. This leads to a strong decrease in early embryo quality.5 This study proposed that supplementation with 5-MTHF, associated with vitamins B3, 6, and 12, and zinc (cofactors of the 1-CC), and N-acetylcysteine, for 4 months for MTHFR carriers, male or female, before starting another ART attempt.
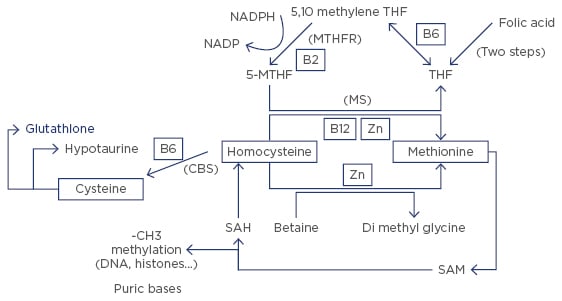
Figure 2: The one carbon cycle (1-CC).
THF: tetrahydrofolate; 5-MTHF: 5-methyltetrahydrofolate; NADP: nicotinamide adenine dinucleotide phosphate; NADPH: NADP reduced form; SAM: S-adenosylmethionine; SAH: S-adenosylhomocysteine.
A total of 31 patients fulfilled all of the 1-CC protocol requirements. Seventeen patients started a pregnancy, including one from a HMZ X HMZ couple, and eight originating from HMZ X HTZ couples. Of all the starting pregnancies, 11 were spontaneous. However, four miscarriages were observed (7–9 weeks) from these 17 pregnancies.
In conclusion, the management of hypo-fertile patients must include a correct evaluation of the MTHFR background of both partners, not just the female partner as in current practice; especially if we consider the possibility that other composite homozygous mutations, the A1298C variation especially, have not been tested here. Nutritional supplementation with 5-MTHF, the metabolite downstream of MTHFR, associated with a support of the 1-CC, is a useful strategy for the isoform carriers. This also suggests that oocyte donation is not an inevitable path for patients with repeated ART failures. This clinical aspect is clinically relevant as current environmental pollution, especially related to the endocrine disruptor chemicals, increases the oxidative stress and exerts a negative pressure on methylation, imprinting, and epigenetics.1








