Pregnancies following assisted reproduction are at higher risk of poorer perinatal outcome.1 Algorithms combining age and various maternal serum hormone concentrations have been used to predict adverse perinatal outcome.2,3 Human chorionic gonadotropin (hCG) is the first known hormonal signal by the embryo, promoting embryo/endometrial molecular cross-talk, cytotrophoblast proliferation, and subsequent invasion.4
In the in vitro fertilisation setting, the exact date of conception is known so it is possible to perform a quantitative βhCG test on a specific pre-determined day: 17 days after the pre-oocyte retrieval trigger injection known as trigger day +17. A positive test can be defined as βhCG >10 IU/L. All cases from the authors’ single centre from fresh stimulated assisted reproductive technology cycles from 2008–2016 inclusive were examined (N=1,846). Only cases in which the βhCG was performed on trigger day +17 were analysed.
Analyses showed that if the serum βhCG concentration was <30 IU/L (n=52), the chances of a continuing pregnancy at 8 weeks gestation was only 2%. If the βhCG concentration was 30–50 IU/L (n=68) it was 24% and if the βhCG concentration was 50–70 IU/L (n=57) it was 33%. This contrasted with 86% if the βhCG concentration was >70 IU/L (n=533, p<0.0001).
In addition to promoting the corpus luteum of early pregnancy, hCG contributes to the maternal tolerance of the embryo via T cell modulation and influences the degree of macrophage migration of the endometrial stromal cells.5,6 However, failure of these mechanisms in early pregnancy may result in miscarriage, which is in keeping with the authors’ observations, and can also reflect poor placentation leading to subsequent adverse perinatal outcomes.7 Hence, an initial low βhCG concentration in a viable pregnancy may be an indicator of subsequent poorer perinatal outcome. To test this, the authors assessed the pregnancy outcomes in these patients.
Overall, only 20% of the cohort with initial βhCG <70 IU/L on trigger day +17 had an ongoing singleton pregnancy (n=36), as opposed to 86% of the women with initial βhCG >70 IU/L (n=533). Only outcomes from singleton pregnancies were examined to exclude potential bias associated with multiple pregnancy (Table 1).
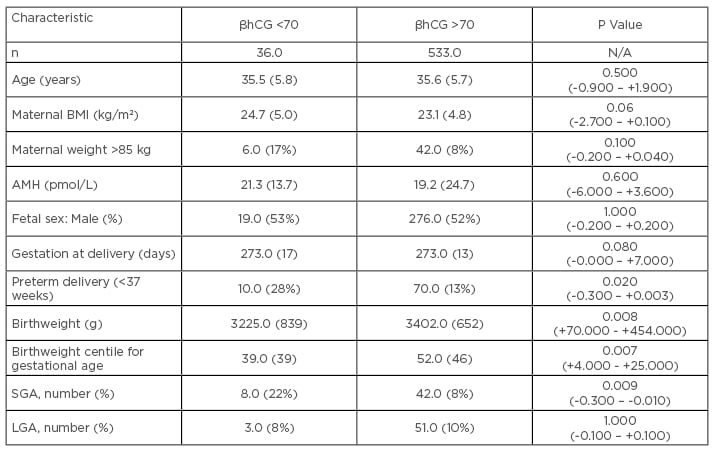
Table 1: Perinatal outcomes of singleton pregnancies.
All continuous variables expressed as median (interquartile range in parentheses). Categorical variables expressed as number (% in parentheses).
Comparisons of continuous data was carried out by the Mann-Whitney U-test; comparisons of categorical data was carried out by Chi-squared analysis (95% confidence intervals of the differences in parentheses).
AMH: Anti-Müllerian hormone; BMI: body mass index; SGA: small for gestational age (birthweight <10th centile for gestational age); LGA: large for gestational age (birthweight >90th centile for gestational age).
There was no difference in maternal characteristics or gestational age at delivery. However, singletons in the low βhCG group were more likely to be born preterm, had a lower birthweight centile for gestational age, and were more likely to be small for gestational age. There were also more stillbirths in the low βhCG cohort (n=3, 8.0%) than in the higher βhCG cohort (n=3, 0.6%; p=0.0040). These data indicate that a critically timed, initial βhCG concentration may be a reasonable guide to early pregnancy viability and allows counselling of patients and management of their expectations accordingly. Furthermore, in viable pregnancies, it identifies a subgroup at the beginning of pregnancy who are at especially high risk of poor perinatal outcome: greater risk of stillbirth, preterm delivery, and being small for gestational age. This subgroup of women merits closer assessment during pregnancy to optimise the ultimate pregnancy outcome.
hCG plays a crucial role in angiogenesis, cytotrophoblast invasion, and immune tolerance,8 so it is perhaps not surprising that it is an early marker of subsequent pregnancy outcome. These observations identify a high-risk group and provide a potential model for future research into implantation and pregnancy development.9








