Abstract
Leydig cell tumours (LCT) are rare sex-cord gonadal tumours that account for less than 5% of testicular neoplasms. They are often discovered incidentally during imaging or as part of infertility evaluations. The authors report a case of a 19-year-old male who presented with complaints of small testes. Apart from raised 17-alpha-hydroxy-progesterone (6 ng/mL), the rest of the hormonal and tumour marker workup was normal. On ultrasound, there was a heterogeneously hypoechoic lesion in the right testis with a hyperechoic rim and peripheral vascularity, on a background of multiple microliths. The lesion was hard on elastography. On MRI, it was hypointense on T2 and short tau inversion recovery (STIR) sequences and was iso-hyperintense on the T1 sequence. There was no diffusion restriction. The patient underwent right orchidectomy. Histopathology showed features of LCT. Common differentials include seminoma (not markedly hypointense on T2, central vascularity, heterogeneous enhancement kinetics, raised tumour markers), Leydig cell hyperplasia (multifocal, bilateral, and asymptomatic), and testicular adrenal rest tumours in children (associated with congenital adrenal hyperplasia and is bilateral). The key radiological features for diagnosing LCTs are subcentimetric size, well-defined margins, hyperechoic rim with peripheral vascularity on ultrasound, and marked hypointensity on T2 sequences without diffusion restriction.
Key Points
1. Leydig cell tumours (LCT) account for less than 5% of testicular tumours. It is essential to accurately diagnose these tumours and to differentiate them from common testicular malignancies such as seminoma.
2. This is a case report of an LCT in a 19-year-old male, highlighting its imaging features on ultrasound, elastography, and multiparametric MRI. It also explains how to distinguish it from common differentials, such as seminoma.
3. LCTs are solitary, subcentimetric homogenous lesions that are hypoechoic on ultrasound with a hyperechoic rim and peripheral vascularity. It is hard on elastography, and is hypointense on T2 sequences with homogenous enhancement.
INTRODUCTION
Leydig cells are found in the testes, specifically in the interstitial space between the seminiferous tubules.1 They are responsible for secreting testosterone, which has a paracrine effect on the seminiferous tubules, thereby contributing to spermatogenesis.1,2 Leydig cell tumours (LCT) are classified as sex-cord gonadal tumours and account for less than 5% of testicular neoplasms.2 Although these tumours are rare, they are the most common type of sex-cord stromal tumours. LCTs are often discovered incidentally during imaging studies or as part of evaluations for infertility. Ultrasound is the primary imaging technique used to assess testicular lesions. Additionally, multiparametric MRI can help differentiate LCTs from other benign conditions and germ cell tumours.
CASE PRESENTATION
A 19-year-old male with short stature presented with complaints of small testes. There was no significant past medical or surgical history. On examination, there were no palpable lesions in the scrotum. Apart from raised 17-alpha-hydroxy-progesterone (6 ng/mL), the rest of the hormonal and tumour marker workup was normal. On ultrasound, there were multiple non-grouped microliths in the right testis. There was a 7×4 mm heterogeneously hypoechoic lesion in the right testis with a hyperechoic rim. It had predominantly peripheral vascularity (Figure 1). The lesion was hard on ultrasound elastography (Figure 2). On MRI, it was hypointense on T2-weighted and short tau inversion recovery (STIR) sequences. It was iso-hyperintense on T1-weighted sequences. There was no diffusion restriction (Figure 3). The patient underwent right orchidectomy. Histopathology showed features of LCT. The patient encountered no complications during the surgery or in the ward course. He was discharged after a few weeks and asked to follow up on an outpatient basis regularly.
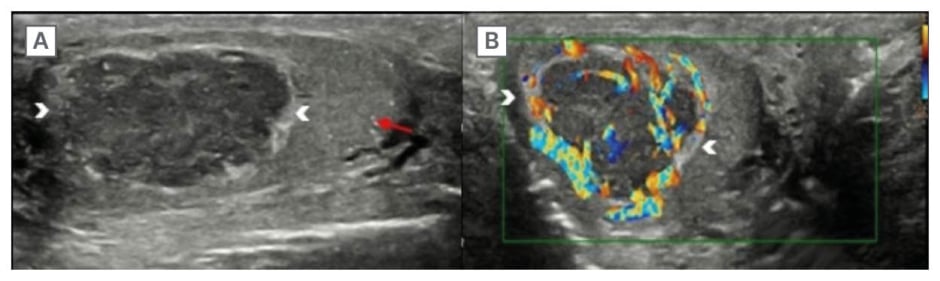
Figure 1: Ultrasound of the testis shows a hypoechoic lesion with hyperechoic rim and peripheral vascularity.
Ultrasound of the right testis (A) with a linear probe, in a longitudinal section, shows a well-defined, heterogeneously hypoechoic lesion with a hyperechoic rim (arrowheads). There are multiple non-grouped microliths (red arrow). On colour Doppler (B), the vascularity is predominantly peripheral (arrowheads).
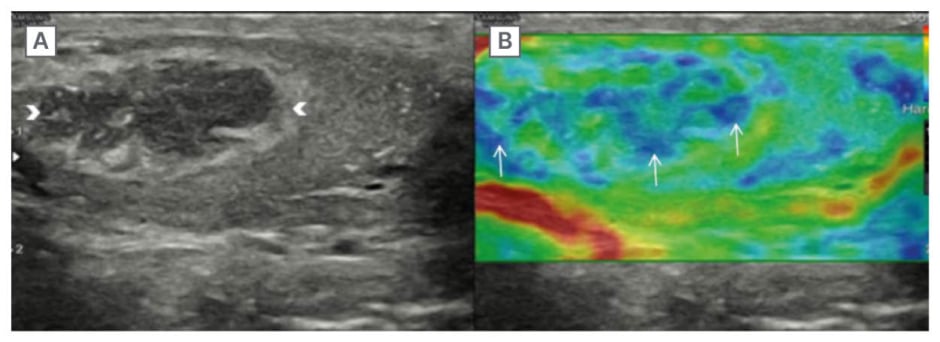
Figure 2: The lesion is stiff on elastography.
Ultrasound of the right testis in B mode (A) and elastography (B) shows that the lesion (white arrowheads) is stiff on elastography (white arrows), depicted by blue colour, which corresponds to ‘hard’ on the scale.
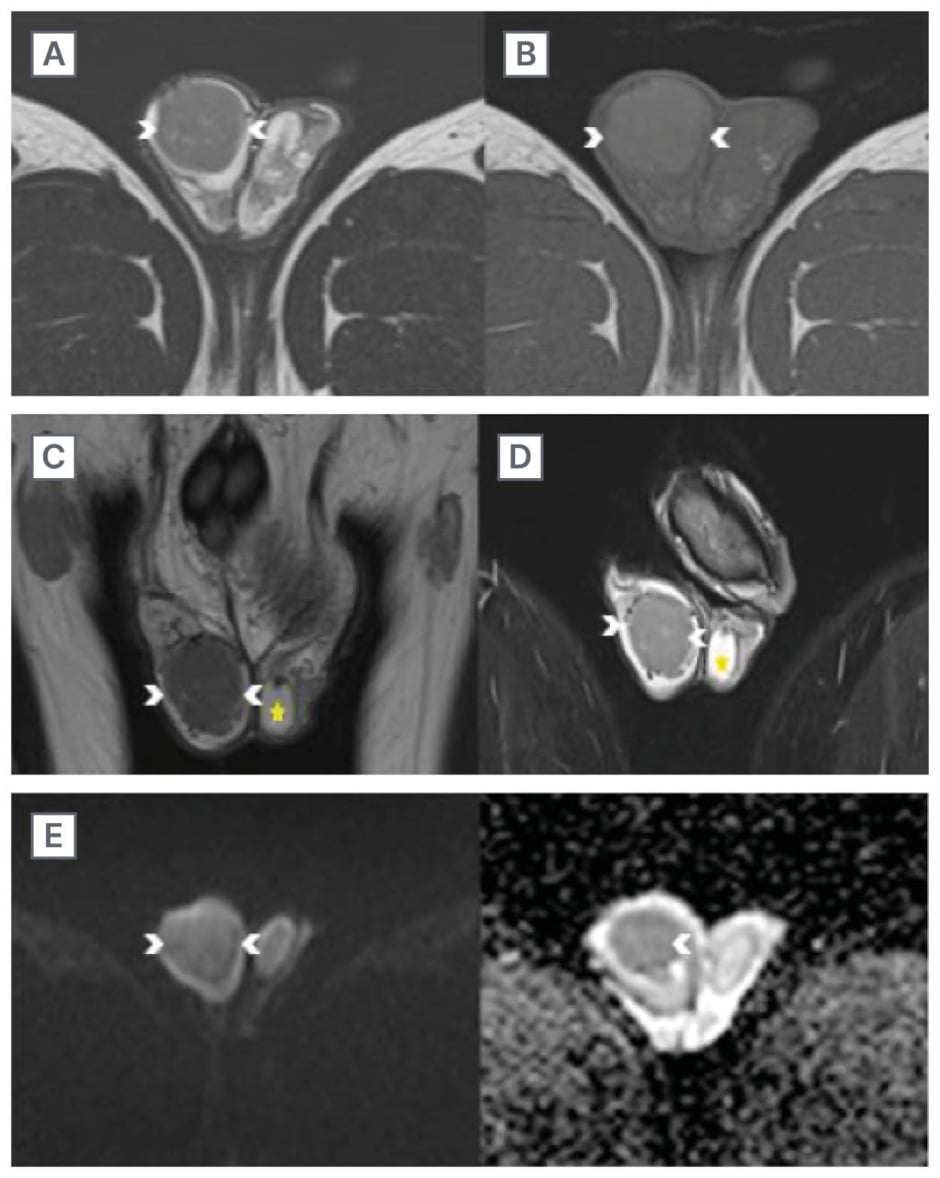
Figure 3: The lesion is hypointense on T2 and short tau inversion recovery, iso-hyperintense on T1 without diffusion restriction.
Axial (A) and coronal (C) T2, and coronal STIR (D) sequences of the MRI of the testes show a well-defined hypointense lesion in the right testis (arrowhead). On the axial T1 (B) sequence, the lesion is iso-hyperintense (arrowhead). The lesion (arrowheads) shows no diffusion restriction (E).
DISCUSSION
LCTs account for approximately 22% of non-palpable testicular nodules.2 They exhibit a dual frequency peak, with one peak occurring in childhood (between the ages of 5–10 years) and another in adults (beyond the third decade).2,3 In children, the most common presentation is precocious puberty, which is caused by the androgen production from these tumours.3 In adults, however, androgen production is less readily detected and is often discovered incidentally or during infertility evaluations. Many patients with LCTs also experience gynaecomastia. Although there is no specific biomarker for LCT, serum markers such as alpha-fetoprotein, beta-human chorionic gonadotropin, and lactate dehydrogenase are typically measured to rule out germ cell tumours, which usually present with elevated tumour marker levels. In contrast, levels in LCTs are generally negative.2 While most cases are unilateral, bilateral occurrences have been reported in about 3% of cases.4 The majority of these tumours are benign, and metastasis is the only reliable criterion for diagnosing malignancy. Additionally, there is a strong association between LCTs and Klinefelter syndrome.2,5
LCTs are usually smaller than 1 cm, with a median size of about 7 mm. Macroscopically, these tumours exhibit a golden–brown colour.2 Due to the ease of access, ultrasound is typically the preferred initial imaging modality; it is also less expensive than other cross-sectional imaging techniques, such as MRI. On ultrasound, LCTs appear as homogeneously hypoechoic lesions with a well-defined interface with the surrounding testicular tissue.2 In some cases, a hyperechoic rim may be observed.3 Although microlithiasis is rare in LCTs, if present, it tends to be non-grouped. Colour Doppler imaging reveals peripheral vascularity, often with a prominent feeding vessel. In contrast-enhanced ultrasound (CEUS), there is centripetal filling with homogeneous enhancement. This enhancement pattern distinguishes LCTs from seminomas, which typically exhibit radial or trans-lesional vascularity.2 It is important to note that this differentiation may be challenging in smaller lesions. In paediatric cases, central vascularisation may be observed.3 On elastography, LCTs appear stiff.2,6
Multiparametric MRI is valuable for further characterisation of these tumours. On T2-weighted MRI, LCTs are usually hypointense compared to the surrounding parenchyma, which is hyperintense.2,6,7 They tend to be iso-hypointense on T1-weighted sequences. The fibrous capsule may appear hyperintense on T2-weighted images. Additionally, areas of hyperintensity within the lesion may indicate the presence of a central scar.4,6 Haemorrhage, necrosis, and fat are not typically associated with LCTs. While the testis has inherent diffusion restriction, LCTs themselves do not exhibit this restriction. The enhancement pattern often shows rapid wash-in and prolonged washout, indicating significant and sustained enhancement, which can be attributed to the abundant vascularisation surrounding and within the LCTs.2,4,6,8 This vascularisation is linked to a high expression of vascular endothelial growth factor derived from endocrine glands.8
The primary differential diagnosis for an LCT is a germ cell tumour, such as seminoma. In cases of germ cell tumours, serum markers are typically elevated. Seminomas usually exhibit blurred margins with the surrounding tissue and are not significantly hypointense on T2-weighted imaging. Additionally, seminomas are characterised by grouped microlithiasis. Their vascularity is often radial and translesional, and they display heterogeneous kinetics on CEUS.2 The key differences between LCT and seminomas have been listed in Table 1.
Leydig cell hyperplasia is a condition characterised by bilateral and multifocal lesions, where patients typically remain asymptomatic. This contrasts with LCTs, which may present with endocrine abnormalities such as gynecomastia.9 Notably, lesions associated with Leydig cell hyperplasia do not exhibit vascularity.
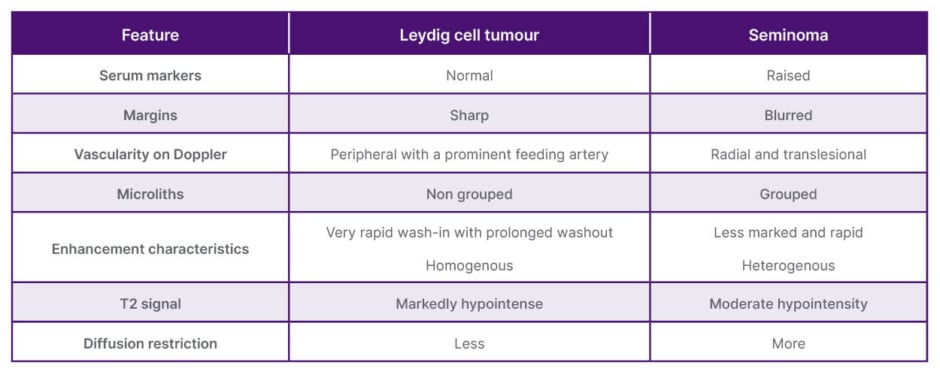
Table 1: Differences between Leydig cell tumour and seminoma.
In children, one of the most common differential diagnoses is testicular adrenal rest tumours (TART), which are often associated with congenital adrenal hyperplasia and tend to be bilateral. On ultrasound, TART lesions can appear either hyper- or hypoechoic and do not have a hyperechoic rim.3 It is also crucial to screen the adrenal glands in these cases.
For small LCTs, testis-sparing surgery (TSS) is recommended. Prompt diagnosis is essential to prevent the need for orchidectomy.10,11 Despite the variety of imaging findings, the specificity of imaging modalities is low, especially for small tumours; therefore, an intraoperative frozen section examination is essential before proceeding with TSS.8,12 Microsurgery is particularly important for cases with bilateral tumours and in patients with solitary testis, as it is more cosmetically appealing. The success of microsurgery can be attributed to the presence of a pseudocapsule, which helps ensure negative tumour margins.12 Metastatic LCT generally shows a poor response to adjuvant chemoradiotherapy. Follow-up imaging is recommended to monitor for recurrence or metastasis. Studies indicate that long-term follow-up shows a disease-free status in all patients, supporting the idea that conservative management is safe for LCTs. However, there is no significant improvement in fertility potential for patients treated for LCT.5 This article serves as a comprehensive repository, meticulously illuminating the imaging characteristics of LCT. Beyond detailing the diagnostic insights offered by ultrasound and MRI, it further accentuates the emerging role of elastography, enriching the discourse with advanced perspectives on its application. The limitation of this article is that it only describes a single case report.
CONCLUSION
LCTs are solitary, subcentimetric, homogeneous hypoechoic lesions seen incidentally or in patients with gynaecomastia and Klinefelter syndrome with negative tumour markers. There can be a hyperechoic rim with a peripheral vascularity. On MRI, it is hypointense on T2 sequences with no diffusion restriction. There is homogeneous enhancement with a rapid wash-in and prolonged washout. Although these imaging features may favour the diagnosis of LCT, a frozen section is mandatory prior to microsurgery.
Patient Perspective
I was really scared when I was told about cancer in my testis and how it would affect my life. However, I was reassured by the hospital team, and I feel perfectly alright post-surgery. Although it was a rough patch in my life, I am glad, as it could have been something worse. I am thankful to the doctors for diagnosing the condition and surgically removing it.
Informed Consent
Written informed consent has been obtained from the patient for writing the manuscript. There is no identifiable information in the manuscript that could reveal the identity of the patient.