Abstract
Cholangiocarcinoma is a rare primary malignancy of the biliary tree, which usually presents late in the course of disease with jaundice, upper right quadrant pain, and cachexia. They frequently metastasise in the lungs, liver, bones, adrenals, peritoneum, and retroperitoneal lymph nodes. The incidence of cutaneous dissemination from cholangiocarcinoma is extremely rare, with the scalp being the commonest distant site of skin metastasis. The authors report the case of a 44-year-old female with Stage IV hilar cholangiocarcinoma, who presented primarily with tender facial swelling, prompting investigation and subsequent diagnosis. To the authors’ knowledge, this case is the first report of a cholangiocarcinoma presenting as facial metastasis. It highlights the need for early characterisation of cutaneous lesions, which are likely to be of neoplastic origin using histology, immunohistochemistry, and PET-CT scans, and reminds that biliary tree neoplasms are possible primary malignancies in cases of skin metastasis, especially in the head and neck region.
Key Points
1. Cholangiocarcinoma is a rare primary malignancy of the intrahepatic or extrahepatic biliary tree, forming <2% of all primary cancers. Rarely, cholangiocarcinomas may present as isolated or multifocal cutaneous metastasis.2. Cutaneous metastases arising from visceral malignancies are extremely rare, with overall incidence between 0.4% and 10.0%; of these, only 0.8% of cases present with cutaneous deposits as the first sign of malignancy.
3. Bile duct malignancy may be considered when addressing cutaneous metastasis of unknown origin, through histological and immunohistochemical analysis backed by clinical and radiological findings.
INTRODUCTION
Cholangiocarcinoma is a malignancy of the intrahepatic or extrahepatic (perihilar or distal) biliary tree, forming less than 2% of all primary cancers.1,2,3 They have a definite male preponderance, with peak incidence in the seventh decade of life.2 Clinically, patients frequently present with jaundice, right hypochondriac pain, and weight loss. However, more often than not, the tumour is locally advanced or metastasised at detection, and patients miss the window for radical surgery,1,2 which is the only potential curative treatment known.3
While the usual sites of spread from a primary cholangiocarcinoma are lungs, liver, bones, adrenals, peritoneum, and retroperitoneal lymph nodes,1,3 the authors’ survey of existing literature yielded a few reports of cholangiocarcinomas presenting as skin metastasis,1-6 most of which already showed extensive visceral metastasis at diagnosis.6 However, cholangiocarcinoma presenting as facial swelling is previously unreported.
CASE REPORT
A 44-year-old female reported in February 2020 with a diffuse tender swelling over the right maxillary and zygomatic areas, of 45 days duration. It was associated with pain and watering from their right eye. There was no history of trauma, fever, pre-existing skin conditions, tuberculosis, or diabetes. Ultrasonography showed diffusely thickened hyperechoic subcutaneous lesion, with internal and peripheral vascularity on colour Doppler, raising suspicion of infectious or inflammatory aetiology.
A CT scan of the paranasal sinuses was performed due to clinical suspicion of invasive sinus pathology. This showed diffuse ill-defined soft tissue lesions in the subcutaneous and myofascial planes of the right premaxillary, prezygomatic, and frontal regions (Figure 1A–C). On MRI, the lesions had homogeneous T2-weighted (T2W)/short-tau inversion recovery hyperintense (Figure 1D–G) and T1-weighted (T1W) isointense signal characteristics relative to muscles (Figure 1H), with diffusion restriction (Figure 1I and J) and heterogeneous post-contrast enhancement (Figure 1K–O). These findings indicated a possible neoplastic infiltrative lesion. For confirmation and further management, the patient was advised biopsy correlation, which was refused, and the patient took discharge, against medical advice.
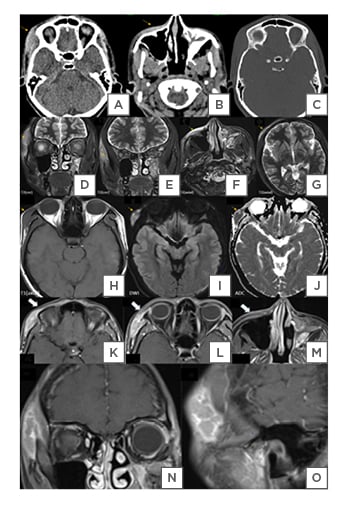
Figure 1: A CT scan of the paranasal sinuses
A) A CT scan of the paranasal sinuses showing ill-defined iso- to hyperdense lesion in the skin, subcutaneous, and myofascial plans and B) pre-maxillary regions. B) Incidentally detected, fibrous dysplasia of the left maxillary sinus. C) No bony erosion can be seen on the bone window.
D and E) T2 hyperintense infiltrative lesion in the right zygomaticotemporal, F) maxillary, and G) frontal soft tissues.
H) Lesions appear to be isointense to the muscle on T1-W, with I) diffusion restriction, and J) corresponding low ADC values.
K–O) Post-contrast MRI sequences show the heterogeneous enhancement of these lesions (the arrow), suggesting neoplastic cutaneous lesions.
ADC: apparent diffusion coefficient; T1-W: T1-weighted.
In May 2020, the patient returned with 7 days’ history of jaundice, right hypochondriac pain, and another new soft tissue swelling over her right arm. The patient had no history of hepatotoxic drug intake or alcohol use. Hyperbilirubinaemia with increased liver enzymes was present. Viral markers for hepatitis were negative.
Ultrasonography of abdomen and pelvis, which was performed at another centre, showed asymmetric intrahepatic biliary radical dilatation in both lobes of the liver, due to a nearly isoechoic mass at the common hepatic duct bifurcation, which was marginally infiltrating into the cystic duct. The common bile duct (CBD) measured 10 mm in diameter. Multiple periportal lymph nodes were seen, the largest measuring around 16 mm.
A triple phase CT scan confirmed the presence of an infiltrative enhancing soft tissue density mass at the common hepatic duct bifurcation (Figure 2A–D), extending into proximal CBD and distal cystic duct, measuring about 39x32x31 mm (transverse x craniocaudal x anteroposterior). Upstream dilatation of the right and left hepatic ducts and intrahepatic biliary radical were noted. Periportal and retroperitoneal lymphadenopathy were present. Enlarged periportal lymph nodes were compressing the portal vein extrinsically. Right paraspinal soft tissue lesions were detected at the ninth and tenth thoracic vertebral levels, suspicious of metastatic deposits. Magnetic resonance cholangiopancreatography confirmed the findings of CT and ultrasonography. The mass had T1-W hypointense and T2-W hyperintense (Figure 2E and F) signal relative to liver, with restricted diffusion. Abrupt cut off of right hepatic duct was seen (Figure 2G and H). It terminated into an intraluminal soft tissue component measuring 7×3 mm in the mid-portion of CBD (Figure 2F), causing a meniscus sign (Figure 2H). Pancreatic portion of CBD was normal. The mass at porta hepatis was compressing the portal vein (Figure 2E) and distal cystic duct, which showed abrupt cut off (Figure 2F and H).
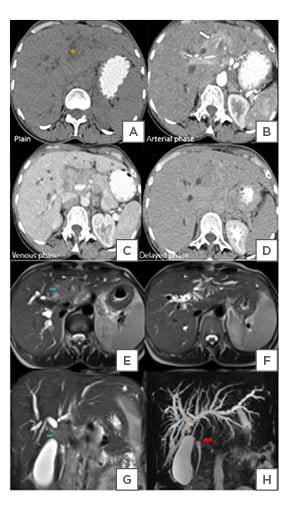
Figure 2: A triple phase CT scan confirmed the presence of an infiltrative enhancing soft tissue density mass at the common hepatic duct bifurcation.
A) A Contrast-enhanced CT scan of the abdomen showing an infiltrative soft tissue mass (star) at the bifurcation of CHD. B) It shows heterogeneous enhancement (arrow) in the arterial phase and related to the right and left hepatic ducts. C and D) The mass is hypoattenuating to the liver in venous and delayed phases. C) The common bile duct is compressed and not well visualised.
E and F) An MRCP showing a heterogeneous T2-W hyperintense mass at the CHD bifurcation (blue arrow). G and H) Abrupt cut-off (star) and non-visualisation of CHD by the mass, which is likely to involve the distal cystic duct. F) It is extending to the CBD, causing H) meniscus sign (red arrow). The rest of the distal is normal. E–H) Asymmetric upstream dilation of the right and let hepatic ducts and IHBR due to obstruction by the mass.
CBD: common bile duct; CHD: common hepatic duct; IHBR: intrahepatic biliary radicals; MRCP: magnetic resonance cholangiopancreatography; T2-W: T2-weighted.
A biopsy of the mass showed central vein and portal triad, with inflammatory cells, suggesting triaditis. Dense fibrocollagenous stroma infiltrated by discrete tumour cells was observed. The tumour cells showed pleomorphic nuclei and hyalinised collagen, with occasional ill-defined glandular differentiation with extracellular mucin. Hepatocytes showed cholestasis, focal steatosis, and regenerative changes. These findings were positive for poorly differentiated sclerosing cholangiocarcinoma.
A PET-CT performed in June 2020 detected fluorodeoxyglucose-avid visceral metastasis (Figure 3) in Segment II of the liver, left suprarenal gland; nodal metastasis in left supraclavicular, retrosternal, mediastinal, right paravertebral, mesenteric, retroperitoneal, pelvic, and bilateral inguinal regions; skeletal metastasis in cervical–thoracic–lumbar vertebrae, sacrum, bilateral pelvic bones, femurs, humeri, scapulae, ribs, and sternum; and subcutaneous or myofascial metastasis in frontal scalp, right frontotemporal region, right cheek, and both arms.
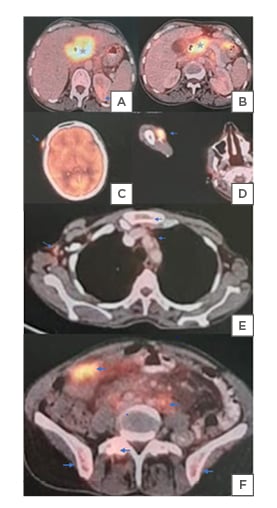
Figure 3: A PET-CT detected fluorodeoxyglucose-avid visceral metastasis.
A) An FDF-PET-CT scan showing cholangiocarcinoma primary (star) at CHD bifurcation, encasing the right and involving the left hepatic ducts and B) CBD. A) Metastatic deposits (blue arrow), as shown, are left suprarenal metastasis.
C) FDG-avid cutaneous metastasis in the right frontal scalp and D) right arm.
E) Mediastinal, retrosternal, retroperitoneal, mesenteric, and F) pelvic nodal metastasis.
E) Skeletal metastasis in sternum, vertebrae, and F) iliac bones.
CBD: common bile duct; CHD: common hepatic duct; FDG: fluorodeoxyglucose.
A final diagnosis of hilar cholangiocarcinoma, with first order right hepatic duct and second order left hepatic duct involvement, and hepatic parenchymal infiltration was reached: Bismuth-Corlette classification Type IV. Regional and distant lymph nodal, hepatic, adrenal, skeletal, and cutaneous metastasis indicated Stage IVB cancer.
The patient underwent palliative extrahepatic biliary drainage, with placement of two metallic biliary stents, followed by four cycles of chemotherapy with folinic acid plus fluorouracil plus oxaliplatin, and is being maintained on oral capecitabine.
DISCUSSION
cutaneous metastases arising from visceral malignancies are extremely rare, with studies reporting variable overall incidence between 0.4–10.0%.2,3,7 Amongst these, a mere 0.8% of cases presented with cutaneous deposits as the first sign of malignancy.6 Excluding malignant melanoma and lymphoma, breast cancer (36.2%), followed by cancers of the lungs (16.3%), colorectal (11.3%), oral mucosa (7.8%), stomach (7.1%), liver (2.8%), oesophagus (2.1%), and kidney, ovary, prostate, and bladder (<1%) showed higher propensity to metastasise to the skin.3,7 Overall, the chest, abdomen, and scalp were the predominant sites for metastasis.7 This is theorised to be due to the dissemination of tumour cells via valveless vertebral venous plexus communicating segmentally with veins of thorax, abdomen, pelvis, and the intracranial dural sinuses.4
Clinically, cutaneous metastasis most commonly present as painless nodular lesions, although macules, plaques, ulcers, erythema,3scarring alopecia, cutaneous horns, and pyoderma gangrenosum-like lesions have also been described.7
Diagnosis of an occult primary tumour from a cutaneous metastasis is possible by histological and immunohistochemical analysis. Typically, tumour cells spare the epidermis, while infiltrating the dermis and subcutaneous tissues. Immunostaining of a metastatic deposit from bile duct malignancy will be cytokeratin-7 positive, cytokeratin-20 positive, or CDX2 negative.8
CUTANEOUS METASTASIS IN CHOLANGIOCARCINOMA
Cholangiocarcinomas disseminate by seeding at the site of percutaneous biliary drainage over thorax (30.3%) or abdomen (20.0%) through catheter tracts, and by metastasising to distant sites, commonly scalp (12.6%), back, or thigh.1,6 Liu et al.1 systematically analysed reports of cholangiocarcinoma presenting with cutaneous metastasis from 1978 to 2014, which yielded only 30 cases from 21 studies. Amongst these, skin lesions were the first sign of the malignancy in 26.7% cases. The cases had a median age of 60 years at diagnosis and definite male predilection. Further, solitary cutaneous metastasis at diagnosis and male sex showed poor outcome. Median overall survival after cutaneous metastasis in cholangiocarcinoma was just 4 months.1 The poor prognosis was likely as the majority of such cases already had extensive visceral metastasis (73.8%) at diagnosis.6 These findings underscore the importance of early diagnosis of cholangiocarcinoma and the high degree of suspicion required when approaching suspicious skin lesions.
CHALLENGES IN IMAGING OF CUTANEOUS METASTASIS
When imaging a cancerous-looking skin or soft tissue lesion, primary skin malignancies (e.g., squamous cell skin cancer, basal-cell carcinoma, melanoma, etc.) and lymphomas are the usual suspects, while metastasis is scarcely considered. Moreover, benign, premalignant, and infectious or inflammatory lesions may mimic a neoplasm, confounding diagnosis. Neoplastic cutaneous lesions may have been incidentally detected, or alternatively can be vague, even when they are the lesions of interest, when located at the edge of an image. Hence, multimodality imaging using CT scans, magnetic resonance studies and PET-CT are useful for accurate diagnosis, staging, treatment planning, and assessment of therapeutic response. CT scans have the advantage of being widely available, cost-effective, and are able to detect pulmonary metastases. Meanwhile, anatomical resolution of superficial tissues provided by MRI is superior. PET-CT is most useful in detecting micrometastasis, indeterminate metastatic nodes, and subtle tumour recurrence.5
CONCLUSION
Cholangiocarcinoma is a rare primary malignancy, which may rarely present as isolated or multifocal cutaneous metastasis. Therefore, probability of bile-duct malignancy should also be kept in mind when addressing cutaneous metastasis of unknown origin. This can be done by histological and immunohistochemical analysis, backed by clinical and radiological findings.
This case of a 44-year-old female with Stage IVB hilar cholangiocarcinoma, first presenting with painful facial swelling, truly highlights the occult nature of bile duct malignancy, its variable presentation, and the clinical vigilance required for its diagnosis.







