Meeting Summary
Dr Oaknin welcomed the delegates to the symposium and presented the objectives and agenda for the meeting as well as a case study presentation. Dr Birrer presented on the current landscape of platinum-sensitive recurrent ovarian cancer (PSR OC), including USA and European treatment guidelines. He highlighted data from three landmark Phase III studies that demonstrated the efficacy of platinum-based doublet therapy for patients with PSR OC. The role of cytoreductive surgery is still being debated but bevacizumab and a newer anti-angiogenic agent, cediranib, may both extend progression-free survival (PFS) in these patients. He predicted that new combinations of therapies will be tested. Prof Ledermann followed with a presentation on the role of poly(ADP-ribose) polymerase (PARP) inhibitors, olaparib, niraparib, and rucaparib, in the treatment of PSR OC. The largest increase in PFS is seen in patients with platinum-sensitive BRCA-mutant tumours but there is also significant benefit over placebo in groups of patients with platinum-sensitive BRCA wild-type tumours. Prof McNeish discussed the challenge of identifying the 30% of women with high-grade serous OC who would respond to treatment with a PARP inhibitor even though their tumour does not carry a BRCA mutation. Loss of heterozygosity (LOH) is a key indicator of homologous recombination deficiency (HRD) but current tests miss some women who would benefit from treatment. Dr Lorusso concluded the meeting by exploring future directions for research into PARP inhibitors, such as whether they should be used in the front-line setting and as single agents rather than in combination with chemotherapy. New combinations with anti-angiogenic and immune-oncology agents show promise, as does the potential for retreatment with a different PARP inhibitor.
Introduction and Case Study Presentation
Doctor Ana Oaknin
Dr Oaknin opened the symposium and presented a case study of a woman with high-grade serous ovarian cancer. The patient received primary debulking surgery followed by first-line combined chemotherapy of carboplatin and paclitaxel and had a complete clinical remission. At 16 months, she was considered to have platinum-sensitive relapse, an unknown BRCA status, and was not a candidate for secondary cytoreductive surgery. Dr Oaknin asked the audience what they would do next for this patient:
- Recommend further genetic testing before deciding on a treatment course?
- Treat with platinum-based chemotherapy?
- Treat with non-platinum-based chemotherapy?
- Treat with platinum-based chemotherapy plus bevacizumab?
- Treat with platinum-based chemotherapy followed by PARP inhibitor maintenance therapy?
In response to the clinical case study, approximately 32% of the audience said they would treat the patient with PARP inhibitor maintenance therapy after platinum-based chemotherapy. At the end of the symposium, that figure had risen to 42%. Additionally, at the start of the symposium, 32% of respondents in the audience said they routinely requested genetic testing when selecting treatments for patients with ovarian cancer, while 15% did not; however, slightly over half of the audience (53%) did not treat patients with ovarian cancer.
The Current Landscape of Platinum-Sensitive Relapsing Ovarian Cancer: Perspectives from Europe and the USA
Doctor Michael J. Birrer
Ovarian cancer is a global health burden and is diagnosed in approximately 250,000 women annually worldwide; the majority of patients present with advanced-stage disease.1,2 All types of ovarian cancer are treated with surgery and chemotherapy, apart from very early ovarian cancer, for which chemotherapy may not be required.2 Ovarian cancer is highly sensitive to chemotherapy, and 80% of patients respond to treatment.3 However, a large proportion of patients also relapse and eventually develop drug-resistant disease.3 Indeed, overall survival (OS) rates have not significantly increased over the last 30 years and approximately 140,000 patients die annually because of this disease.1,4 Ovarian cancer represents the highest case-fatality rate for gynaecological cancers in the world.3,4 Approximately three-quarters of patients with advanced stage cancer have platinum-sensitive disease. Both the National Comprehensive Cancer Network (NCCN) and The European Society for Medical Oncology (ESMO) guidelines recommend platinum-based combination chemotherapy for the treatment of PSR OC (Box 1).5-7 The ESMO guidelines advise that, if available, bevacizumab in combination with carboplatin and paclitaxel should be used as a targeted therapy for patients with advanced ovarian cancer with poor prognostic features, including suboptimal debulking. Furthermore, bevacizumab is recommended in combination with gemcitabine and carboplatin chemotherapy in patients with platinum-sensitive relapsed ovarian cancer who have not received bevacizumab previously.6 The NCCN guidelines recommend secondary cytoreduction as a valid option for patients with PSR OC.5 However, the ESMO recommendations consider secondary cytoreduction surgery to be controversial.6
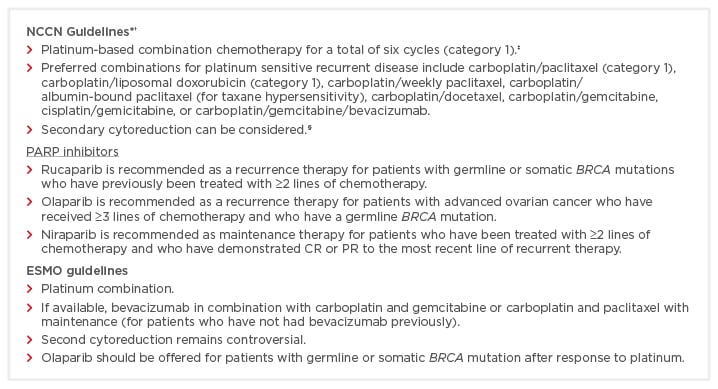
Box 1: The NCCN and ESMO guidelines for the treatment and management of PSR OC.5-7
CR: complete response; ESMO: European Society for Medical Oncology; NCCN: National Comprehensive Cancer Network; PARP: poly(ADP-ribose) polymerase; PR: partial response; PSR OC: platinum-sensitive recurrent ovarian cancer.
*The NCCN guidelines referred to here are the most recent version, which has been updated since this symposium took place. The guidelines have been updated as a result of recent evidence and legislation; for instance, rucaparib has recently been approved in the USA for maintenance treatment of recurrent ovarian cancer; †All recommendations are category 2A unless otherwise indicated; ‡For patients who cannot tolerate combination therapy, the preferred single agent is carboplatin or cisplatin; §Secondary cytoreduction can be considered in patients with recurrent ovarian cancer without ascites who have recurred more than 6–12 months since initial chemotherapy was completed and in whom the disease is amenable to complete resection (i.e, has an isolated focus or limited foci).
Three landmark Phase III studies have demonstrated the efficacy of platinum-based doublet therapy for patients with PSR OC.8-10 ICON48 is the oldest of the three studies; patients were randomised to conventional platinum chemotherapy or a combination of paclitaxel and platinum chemotherapy. Highly statistically significant outcomes were observed for PFS in favour of the combination arm (median PFS: 13 months versus 10 months; hazard ratio: 0.76; 95% confidence interval [CI]: 0.66–0.89; p=0.0004).8 The combination arm also showed significant results for OS (median OS: 29 months versus 24 months; hazard ratio: 0.82; 95% CI: 0.69–0.97; p=0.02).8 The second study, AGO-Ovar 2.5,9 randomised patients to carboplatin or carboplatin and gemcitabine. The median PFS was significantly longer for the combination arm at 8.6 months compared to 5.8 months (hazard ratio: 0.72; 95% CI: 0.58–0.90; p=0.0031); however, this did not translate into an OS advantage.9 The final study, GCIG-CALYPSO,10 compared carboplatin (AUC 5) plus pegylated liposomal doxorubicin versus standard carboplatin and paclitaxel in patients with secondary PSR OC. PFS results showed pegylated liposomal doxorubicin in combination with carboplatin to be an efficacious regimen for these patients, with a PFS of 11.3 months compared to 9.4 months in the standard carboplatin and paclitaxel arm (hazard ratio: 0.82; 95% CI: 0.72–0.94; p=0.005), although these results did not translate into an OS advantage.10 Findings from these three studies established platinum doublets as the standard of care for patients with PSR OC. However, would these patients benefit from secondary cytoreductive surgery?
Various studies have addressed the role of secondary cytoreductive surgery in the treatment of PSR OC. DESKTOP III,11 for example, randomised patients to cytoreductive surgery followed by a platinum-based doublet versus a platinum-based doublet regimen alone. An interim analysis showed that patients who underwent cytoreductive surgery demonstrated some improvement in PFS and time to first subsequent therapy.11 However, until OS data become available, the role of cytoreductive surgery in these patients will remain a subject of debate.
Since bevacizumab became available as both first-line therapy and for the treatment of PSR OC, several trials have studied its effects in combination with platinum agents and cytoreductive surgery.12-14 OCEANS12 enrolled PSR OC patients with measurable disease who had not previously received bevacizumab or chemotherapy for their recurrence. They were randomised to receive either bevacizumab (15 mg/kg) until progression along with gemcitabine (1,000 mg/m2) and carboplatin (AUC 4) (n=242), or the same gemcitabine/carboplatin regimen with placebo (n=242). A highly significant improvement in PFS was observed in the group receiving bevacizumab compared to placebo (median PFS: 12.4 months versus 8.4 months; hazard ratio: 0.48; 95% CI: 0.39–0.61; p<0.0001) but there was no significant difference in OS (median OS for bevacizumab: 33.6 months; median OS for placebo: 32.9 months). The most common grade ≥3 adverse event (AE) associated with bevacizumab was hypertension; this occurred in 18.2% of patients receiving gemcitabine/carboplatin/bevacizumab compared to 0.9% of those in the placebo arm.13
A similar study, GOG-213,14 explored the role of bevacizumab in women with recurrent ovarian, peritoneal primary, or fallopian tube cancer and a treatment-free interval ≥6 months. They all received a combination of carboplatin (AUC 5) and paclitaxel (175 mg/m2) and were randomised to receive (n=337) or not receive (n=337) bevacizumab (15 mg/kg) until progression or toxicity precluding further treatment. Results showed a significant improvement in PFS for those receiving paclitaxel/carboplatin/bevacizumab compared to the group receiving paclitaxel/carboplatin only (median PFS: 13.8 months versus 10.4 months; hazard ratio: 0.63; 95% CI: 0.53–0.74; p<0.0001). Median overall survival in the paclitaxel/carboplatin/bevacizumab group was 42.2 months and 37.3 months in the paclitaxel/carboplatin group (hazard ratio: 0.83; 95% CI: 0.68–1.01; p=0.056). A post-hoc sensitivity analysis utilising the actual platinum-free interval, calculated based on the electronic case report forms, resulted in a hazard ratio of 0.82 (95% CI: 0.68–1.00; p=0.0447).14 As in the OCEANS study, grade 3 hypertension was common and affected 12% of the bevacizumab group, compared to 1% in the other study arm.14 Risk of grade 3–4 proteinuria was elevated with bevacizumab (8% versus 0%) but there were no thrombolytic events in either arm. This study also looked at the role of cytoreductive surgery, but those results have yet to be analysed.14
A second anti-angiogenic molecule, cediranib, has also been tested in PSR OC. Cediranib inhibits the VEGF receptor among other targets and, in ICON6,15 was used in combination with platinum-based chemotherapy as maintenance treatment. Patients received up to six cycles of platinum-based chemotherapy and then entered a maintenance phase. They were randomised to receive chemotherapy/placebo treatment and then placebo-only maintenance (n=115; Arm A), chemotherapy/cediranib (20 mg once daily) and placebo maintenance (n=171; Arm B), or chemotherapy/cediranib (20 mg once daily) and cediranib at the same dose as maintenance (n=158; Arm C). The trial’s primary efficacy endpoint was PFS between Arm A and Arm C. The trial was ended early but results showed a significant improvement in PFS for patients taking cediranib in both treatment and maintenance phases compared to those who received placebo in both (median PFS: 11.0 months versus 8.7 months; hazard ratio: 0.56; 95% CI: 0.44–0.72; p<0.0001).15 A similar trend was observed in OS; however, these data are not yet mature, with 52% of patients having died by data cut-off, and are not statistically significant (median OS Arm A: 21.0 months; median OS Arm C: 26.3 months; hazard ratio: 0.77; 95% CI: 0.55–1.07; p=0.11).15
In conclusion, Dr Birrer explained that platinum doublets are the standard of care for the treatment of PSR OC. The role of cytoreductive surgery remains controversial and further studies are needed to determine whether it is beneficial for these patients. Anti-angiogenic therapy is also effective for maintaining PFS in PSR OC, but there are other combination therapies to be explored.
Role of PARP Inhibitors in Advanced Ovarian Cancer: Where Are We Now?
Professor Jonathan Ledermann
PARP inhibitors are a novel targeted therapy and work by inducing cell death when homologous repair is deficient, which typically occurs in cancer cells harbouring deleterious BRCA1/2 mutations.16 When PARP enzymatic activity is inhibited, single-strand breaks are converted to double-strand breaks during the replication process, and these cannot be repaired by homologous recombination (HR) due to mutations in BRCA1/2.16 The resulting genomic instability leads to cell death. In serous ovarian cancer, several mechanisms might lead to deficiencies in HR. The most common are BRCA mutations, which occur in approximately 20% of patients. Most are germline but 6–8% of tumours have somatic BRCA mutations. A number of other mutations and methylations, such as BRCA1 promoter hypermethylation, prevent the HR repair process,17 meaning that, overall, 40–50% high-grade serous OC may be deficient in HR repair.
Prof Ledermann explained that single-agent studies with olaparib, niraparib, and rucaparib have consistently demonstrated positive results in patients with recurrent BRCA-mutated high-grade ovarian cancers.18 However, olaparib efficacy was also shown in patients without a BRCA mutation,19 which led to the examination of PARP inhibitors as maintenance therapy in high-grade serous cancer in both patients with and without BRCA mutations. Several studies have compared PARP inhibitors with placebo as maintenance therapy in patients with platinum-sensitive, relapsed, high-grade ovarian cancer following ≥2 prior lines of platinum-based chemotherapy. In Study 19, for example, Ledermann et al.20 included patients irrespective of BRCA mutation status and randomised them either to 400 mg twice daily (BID) olaparib (n=136) or placebo (n=129). A highly statistically significant response in the primary endpoint of PFS was observed in all patients (median PFS olaparib: 8.4 months; median PFS placebo: 4.8 months; hazard ratio: 0.35; 95% CI: 0.25–0.49; p<0.001). A pre-planned analysis according to BRCA status confirmed the expected but striking PFS improvement in BRCA-mutated cancers (median PFS in olaparib group: 11.2 months; median PFS in placebo group: 4.3 months; hazard ratio: 0.18; 95% CI: 0.10–0.31; p<0.0001).21 Olaparib-treated patients with wild-type BRCA also showed an improvement in PFS (median PFS in olaparib group: 7.4 months; median PFS in placebo group: 5.5 months; hazard ratio: 0.54; 95% CI: 0.34–0.85; p=0.0075), building on efficacy data from other studies19,22 and, indeed, these results were instrumental in the approval of olaparib in the USA.
A second olaparib maintenance study, SOLO2,23 further examined the effect of olaparib tablets (300 mg BID) versus placebo in patients with PSR OC and germline BRCA1/2 mutations. The study met its primary endpoint demonstrating an increase in investigator-assessed PFS with olaparib (median PFS in olaparib group: 19.1 months; median PFS in placebo group: 5.5 months; hazard ratio: 0.30; 95% CI: 0.22–0.41; p<0.0001). This benefit was supported by a significant delay in the median time to first subsequent therapy of 27.9 months for patients taking olaparib versus 7.1 months for those taking placebo.23 This difference of 20.8 months (hazard ratio: 0.28; 95% CI: 0.21–0.38; p<0.0001) is important to patients because it represents the time they are spared chemotherapy.
The NOVA study24 examined niraparib as maintenance therapy in PSR OC. Patients were divided into two cohorts: those with germline BRCA mutations and those without. They were randomised within cohorts either to receive 300 mg daily niraparib or placebo. Significant improvements in PFS were seen with niraparib in both groups regardless of the presence or absence of germline BRCA mutations.24 The median PFS in the cohort with germline BRCA mutations was 21.0 months for those receiving niraparib and 5.5 months for those receiving placebo (hazard ratio: 0.27; 95% CI: 0.17–0.41; p<0.001), and the median PFS in the non-germline BRCA mutation cohort was 9.3 months for those receiving niraparib and 3.9 months for those receiving placebo (hazard ratio: 0.45; 95% CI: 0.34–0.61; p<0.001). Furthermore, an exploratory analysis performed using the Myriad myChoice® (Myriad Genetics, Inc., Salt Lake City, Utah, USA) assay on HRD-positive patients showed significant results with niraparib both in patients with somatic BRCA mutations and in those with BRCA wild-type.25 More surprisingly, efficacy was also shown in HRD-negative patients, highlighting the fact that HRD tests do not accurately predict which patients will respond to niraparib.
Finally, the Phase III trial of rucaparib, ARIEL3,26 enrolled women with platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube cancer. The women had normal CA-125 levels at entry to the trial and were randomised to 600 mg BID rucaparib (n=375) or placebo BID (n=189). Patients were stratified according to HRD as determined by gene mutation status of the tumour tissue. There were three stratification factors: a) mutation in BRCA1 or BRCA2, b) mutation in a non-BRCA gene that was associated with HR, and c) none of the previous mutations. Patients had BRCA wild-type and low or indeterminate LOH. They were also stratified according to their response to platinum and their PFS interval after the penultimate platinum. The primary endpoint was investigator-assessed PFS and the trial used a nested cohort analysis. The BRCA mutant cohort (n=196) was examined first. Median PFS in the patients treated with rucaparib in this cohort was 16.6 months compared with 5.4 months in patients treated with placebo (hazard ratio: 0.23; 95% CI: 0.16–0.34; p<0.0001). Analysis of the cohort with a mutation in a gene associated with HR (n=354) showed a median PFS of 13.6 months in the rucaparib group compared with 5.4 months in the placebo group (hazard ratio: 0.32; 95% CI: 0.24–0.42; p<0.0001). In the overall intention-to-treat population (n=564), median PFS was 10.8 months in the rucaparib group and 5.4 months in the placebo group (hazard ratio: 0.36; 95% CI: 0.30–0.45; p<0.0001).26 The analysis showed that rucaparib is active across all patients with high-grade serous OC responding to platinum (Figure 1).26
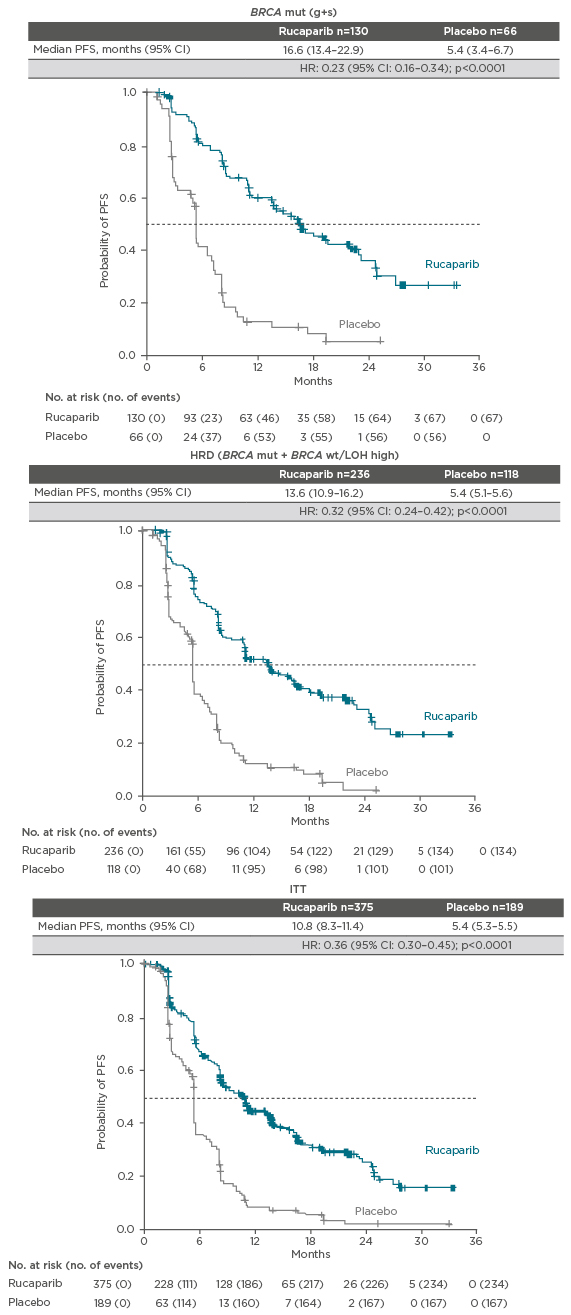
Figure 1: Investigator-assessed PFS in BRCA-mutated patients, HRD (BRCA-mutated and LOH-high) patients and the ITT population in ARIEL3 with rucaparib.26,27
CI: confidence interval; HR: hazard ratio; HRD: homologous recombination deficiency; g+s: germline plus somatic; ITT: intention-to-treat; LOH: loss of heterozygosity; mut: mutation; PFS: progression-free survival; wt: wild-type.
Prof Ledermann described the importance of considering the safety and tolerability of maintenance therapy in PSR OC, as this can have a considerable impact on patients receiving long-term treatment. Low incidences of serious AE were observed in SOLO2,23 NOVA,24 and ARIEL3.26 AE of grade 3–4 in patients receiving olaparib in SOLO2 were nausea (3%; placebo: 0%), fatigue or asthenia (4%; placebo: 2%), vomiting (3%; placebo: 1%), and abdominal pain (3%; placebo: 3%). The most commonly reported haematological AE of grade ≥3 were anaemia (19%; placebo: 2%), neutropenia (5%; placebo: 4%), leucopenia (2%; placebo: 0%), and thrombocytopenia (1%; placebo: 1%).23 Forty-five percent of patients receiving olaparib required dose interruptions (placebo: 18%), with 25% of patients needing dose reductions (placebo: 3%) and only 11% discontinuations due to unacceptable side effects (placebo: 2%).23 The NOVA study24 of niraparib highlighted similar AE to SOLO2, nausea, fatigue, constipation and vomiting, but in addition, hypertension of any grade was observed in 19.3% of patients taking niraparib (placebo: 4.5%). The haematological profile in NOVA differed from that in SOLO2, with thrombocytopenia grade 3–4 seen in 33.5% (placebo: 0.6%) and neutropenia grade 3–4 in 19.6% of patients on niraparib (placebo: 1.7%). Dose interruption was reported in 68.9% of patients on niraparib (5% on placebo), dose reduction in 66.5%, (placebo: 14.5%), and 14.7% discontinued treatment (placebo: 2.2%). The ARIEL3 data for rucaparib showed common side effects included nausea and asthenia, but these were typically low-grade with low rates of grade ≥3 AE reported (3.8% and 6.7%, respectively). A grade ≥3 increase in liver enzymes was seen in 10.5% of patients (placebo: 0%), but this tended to be a transient effect. The most common haematological AE of grade ≥3 reported in patients treated with rucaparib were anaemia (18.8%; placebo: 0.5%) and neutropenia (6.7%; placebo: 1.1%).26 Dose interruption was reported in 63.7% of patients on rucaparib (placebo: 10.1%), dose reduction was needed in 54.7% (placebo: 4.2%), and discontinuation in 13.4% (placebo: 1.6%).26
Study 19 reported in 201120 and the long-term outcome data suggest an overall survival benefit for all patients taking maintenance olaparib versus placebo; however, these results did not reach statistical significance (median OS: 29.8 versus 27.8 months; hazard ratio: 0.73; 95% CI: 0.55–0.96; nominal p=0.025, which did not meet the p<0.0095 required for significance).28 A cohort of approximately 11% of the patients, with and without BRCA mutations, have taken the drug for more than 6 years without evidence of OC recurrence.
Prof Ledermann summarised that PARP inhibitors are suitable for use as both single agents and as maintenance therapy post platinum-based chemotherapy for recurrent high-grade disease. The largest increase in PFS is seen in patients with BRCA-mutated tumours, but there is significant benefit over placebo in patients with platinum-sensitive tumours with and without BRCA1 or BRCA2 germline mutations. Toxicity is generally low grade and a low proportion of patients discontinue treatment with PARP inhibitors. Patients and physicians have an increasing number of choices for therapy.
BRCA and Homologous Recombination Deficiency: To Test or Not to Test, Is That the Question?
Professor Iain McNeish
There are two broad assumptions about the role of PARP inhibition in DNA repair. The first, generally accepted to be true, is that the sensitivity of a tumour to PARP inhibition is a function of defective HR.29 The second, probably not true, is that PARP inhibitors function by blocking the base excision repair pathway.30
So, how is genotypic or phenotypic HRD measured? Prof McNeish described how functional assays of pure DNA damage repair are not suitable for use in clinical trials because they require the ability to keep tumour cells alive in culture. Phenotypic and genomic assays are therefore more useful. Platinum sensitivity is an established phenotypic assay that directly correlates with defective hazard ratio: platinum-sensitive patients are overwhelmingly HR deficient.31 Ledermann et al.20 demonstrated in Study 19 that platinum sensitivity was a suitable surrogate for predicting which patients may benefit from a PARP inhibitor. However, as previously mentioned, patients without BRCA mutations can respond to PARP inhibitors,24,26,32 and relying solely on platinum sensitivity may exclude women who would have benefited from treatment. Genomic assays, such as the Foundation Medicine (Cambridge, Massachusetts, USA) T5 next-generation sequencing assay and the Myriad myChoice® HRD test, can therefore be used to help determine which women may benefit from PARP inhibitor therapy.
Somatic and germline BRCA1/2 mutations occur in approximately 20% of newly diagnosed high-grade serous OC,17 and it is estimated that 50–51% will be HR defective.31,33,34 The challenge is to identify the 30% of women without BRCA mutations who would benefit from PARP inhibitors. However, non-BRCA HR gene mutations are rare, so many genes must be tested in order to determine any abnormalities.32 Importantly, not all gene mutations in the HR pathway result in the same phenotype, and a wide spectrum of sensitivity towards rucaparib, using small-interfering RNA knockouts, was observed previously, even in genes known to be involved in double-strand DNA repair.32
Prof McNeish explained that the genomic damage of BRCA1/2-mutated tumours is primarily represented by LOH, and this can be measured by comprehensive genomic profiling based on next-generation sequencing.35 Tumours can be divided into three subtypes: BRCA-mutated, BRCA wild-type/LOH-high, and BRCA wild-type/LOH-low.35 PARP inhibitors are likely to be effective in the BRCA-mutated cancers; one hypothesis investigated in the multicentre, open-label, Phase II ARIEL2 trial was that patients with BRCA wild-type/LOH-high tumours would also benefit while those with BRCA wild-type/LOH-low tumours would not.
Data from The Cancer Genome Atlas and Australian Ovarian Cancer Study were used to determine a LOH cut-off score of 14% (i.e., ≥14% was considered LOH-high and <14% was considered LOH-low) (Figure 2).38 This score was prospectively applied to ARIEL2 and the results showed a significantly longer PFS for single-agent rucaparib in LOH-high versus LOH-low patients (median PFS: 5.7 months versus 5.2 months; hazard ratio: 0.62; 95% CI: 0.42–0.90; p=0.011).38
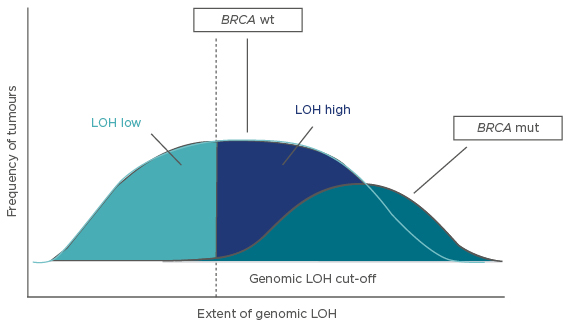
Figure 2: The three molecular subtypes identified, showing the cut-off of 14%* between LOH-low and LOH-high.32
*A cut-off value of 14% was used for ARIEL2 Part 1. A retrospective analysis of the ARIEL2 Part 1 data determined a cut-off of 16% was more optimal; therefore, this cut-off was applied to ARIEL3.36,37
mut: mutant; wt: wild-type; LOH: loss of heterozygosity.
A further analysis of the subset of 117 patients in ARIEL2 who had biopsies at diagnosis and at relapse was conducted. LOH analyses were completed on both biopsy samples, and there was good correlation in that 100 out of 117 tumours were in the same LOH category at both diagnosis and relapse (r=0.86; p<0.0001).38 The remaining 17 tumours (14.5%) had LOH-low at the time of diagnosis but were LOH-high at relapse; no tumour went from LOH-high to low. This demonstrated that LOH scores may evolve as the tumour is exposed to more treatment.38 Interestingly, the RAD51C gene was the most common non-BRCA mutation identified in ARIEL2, resulting in a similar phenotype to BRCA1/2 and response to rucaparib.38 Similarly, in ARIEL3, BRCA wild-type rucaparib-treated patients in the LOH-high subgroup had a longer PFS than patients in the LOH-low subgroup.26 However, some women in the low LOH group also benefited from rucaparib compared with placebo (median PFS: 6.7 months versus 5.4 months; hazard ratio: 0.58; 95% CI: 0.4–0.85; p=0.049), suggesting that the current test does not delineate potential responders to rucaparib sufficiently well. As previously mentioned, similar results were observed in the NOVA study, which found that the benefit of niraparib versus placebo in BRCA wild-type HRD-positive tumours (median PFS: 9.3 months versus 3.7 months; hazard ratio: 0.38; 95% CI: 0.23–0.63; p<0.0001) was greater than in patients with HRD-negative tumours (median PFS: 6.9 months versus 3.8 months; hazard ratio: 0.58; 95% CI: 0.36–0.92; p<0.02) but that patients in both groups benefitted from the treatment.
Prof McNeish continued by explaining that the Myriad myChoice HRD test is more complicated and consists of three elements: LOH, telomeric allelic imbalance, and large-scale state transitions.39 Using an HRD cut-off score of 42, which was also the lower limit of the 95% CI for the LOH score for the BRCA-mutated tumours, the test could differentiate between HR-competent and HRD tumours.39
Prof McNeish noted that while the women included in the NOVA and ARIEL3 studies did very well, there are other women who did not meet the inclusion criteria for these studies who may still benefit from PARP inhibitors as single-agent therapy. Prof McNeish explained that all women with high-grade ovarian cancer should be tested for germline and somatic BRCA1/2 mutations, as well as RAD51C, in order to appropriately identify patients who may benefit the most from single-agent PARP inhibitor maintenance therapy. These studies24,26 have shown that platinum sensitivity matters and LOH analyses may be more sensitive than panel sequencing or methylation analyses for determining which patients benefit most from PARP inhibitors.38 Prof McNeish added that archival tissues can be adequate but fresh biopsies at relapse are preferable.38 Finally, Prof McNeish noted that not all women with platinum-sensitive ovarian cancer want or require maintenance therapy, in which case there is still a role for single-agent PARP inhibitors in the treatment setting; assessing HRD may be invaluable for these patients.
BRCA and Beyond! Future Directions for Enhancing PARP Inhibitor Therapy
Doctor Domenica Lorusso
The clinical utility of PARP inhibitors in BRCA-mutated patients is well established, and their clinical role in non-BRCA-mutated patients is also evident.24,40 However, further development of PARP inhibitor research is required in order to focus on overcoming resistance to PARP inhibitors, possible re-treatment with PARP inhibitors, and the use of PARP inhibitors as a front-line or combination therapy.
Dr Lorusso explained that mechanisms of resistance and their potential impact on retreatment or second-line treatment with PARP inhibitors are important clinical questions. Preclinical observations have demonstrated innate resistance mechanisms, including decreased intracellular availability of PARP inhibitors, increased HR, or epithelial to mesenchymal transition.41-45 Acquired resistance has also been observed in clinical settings, mainly related to the appearance of secondary somatic mutations that can restore BRCA1/2 activity, a possible mechanism of platinum resistance.46-48 This mechanism is suggested to be responsible for 20% of acquired resistance while up to 10% is thought to be related to TP53BPI activity, a protein with opposing activity to BRCA1 in preventing DNA resection and promoting non-homologous end-joining. However, approximately 75% of resistance is due to unknown mechanisms and further investigation is required.49,50 The OReO trial51 is an ongoing Phase III trial of olaparib retreatment following prior PARP inhibitor treatment and complete response or partial response to platinum-based chemotherapy in patients with epithelial ovarian cancer. This trial will help provide insight into the discussed resistance mechanisms.
Dr Lorusso described how the clinical utility of PARP inhibitors as front-line therapy is currently being explored. Patients undergoing treatment for PSR OC will often have been exposed to multiple lines of chemotherapy. The use of PARP inhibitors, which have low toxicity, may allow these patients some respite from chemotherapy and help to increase time to subsequent treatment, thereby allowing adequate recovery from the AE of chemotherapy.18 The SOLO-1 trial52 (Phase III) is investigating olaparib as maintenance treatment in patients with germline BRCA1/2-mutated ovarian cancer, following first-line platinum-based chemotherapy. The PRIMA trial53 (Phase III) is evaluating niraparib as first-line maintenance therapy following platinum-based chemotherapy in patients with high-risk, high-grade ovarian cancer, regardless of HRD mutation. The GOG3005 Phase III trial54 is investigating the PARP inhibitor veliparib in combination with paclitaxel and carboplatin as a first-line treatment and maintenance therapy for advanced ovarian cancer regardless of mutation status.
Another question of clinical importance is the use of PARP inhibitors as combination therapy. PARP inhibitors demonstrate anti-angiogenic activity,55 and preclinical data suggest HR can be suppressed by hypoxia through the downregulation of HR repair proteins such as BRCA1 and RAD51.56,57 In addition, studies have shown sensitivity to PARP inhibitors is enhanced in hypoxic states.58-61 Collectively, such evidence supports the development of PARP inhibitors in combination with anti-angiogenic therapies. Phase I60 and Phase II61 clinical studies have demonstrated improved PFS in patients with platinum-sensitive recurrent disease when treated with combination olaparib and cediranib compared to olaparib alone (median PFS: 17.7 months versus 9.0 months; hazard ratio: 0.42; p=0.005). There are several ongoing Phase III trials investigating PARP inhibitor-based combination therapy. The ICON9 study62 is looking at olaparib with cediranib as maintenance therapy in patients with relapsed PSR OC, following response to platinum-based chemotherapy. The PAOLA-1 trial63 is evaluating combination olaparib/bevacizumab as a first-line maintenance therapy in patients with advanced ovarian cancer. The MITO-25 Phase II trial64 is comparing combination rucaparib/bevacizumab with rucaparib alone or bevacizumab alone as first-line maintenance therapy in patients with advanced ovarian cancer. The Phase I/II ANANOVA trial65 aims to evaluate combination niraparib/bevacizumab in patients with platinum-sensitive ovarian cancer.
Other suggested combination therapies that have shown promise are PARP inhibitors with checkpoint inhibitors. Tumours with deleterious mutations in the DNA repair genes, including BRCA1/2, have a high mutational load and a higher number of protein-coding mutations, a consequence of their inability to repair DNA damage effectively.66 In addition, BRCA1/2-mutated and HRD tumours have been correlated with higher programmed death-ligand 1 (PD-L1) expression and CD8 T cell infiltration that predict PD-L1 monoclonal antibody response.67 A BrKras (BRCA1 null) syngeneic model demonstrated increased tumour infiltration of CD8 T cells and increased survival in response to rucaparib with anti-PD-1 or anti-PD-L1 agents.68 Currently, several ongoing Phase I–III trials are evaluating PARP inhibitors with checkpoint inhibitors in the treatment of ovarian cancer and solid tumours. The TOPACIO open-label trial of niraparib with pembrolizumab in patients with ovarian cancer or triple-negative breast cancer is of particular note.69 Another study of particular relevance is a Phase I/II open-label trial evaluating three doses (400, 500, and 600 mg) of rucaparib in combination with atezolizumab in solid tumours and gynaecological malignancies.70
In summary, Dr Lorusso said PARP inhibitors are changing the natural history of this disease in a substantial number of patients. Further investigations are still required to determine the benefits of monotherapy versus combination therapy, to identify optimal treatment algorithms (first-line, recurrent maintenance or as single agent), and to assess the potential for retreatment with different PARP inhibitors. She said PARP inhibitors offer a good opportunity for patients and that the story is just beginning.








